| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5370894 | 1503921 | 2015 | 13 صفحه PDF | دانلود رایگان |
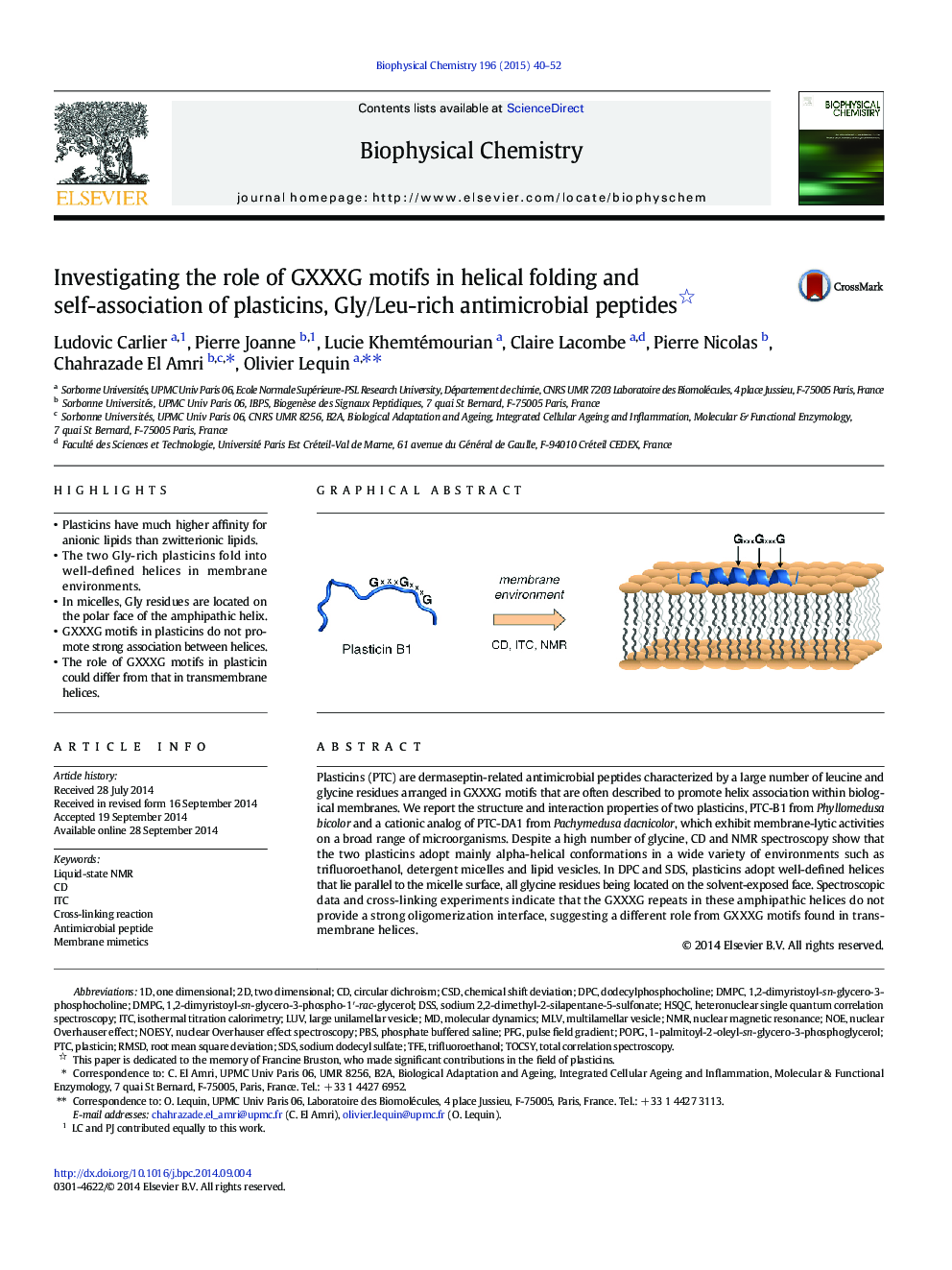
- Plasticins have much higher affinity for anionic lipids than zwitterionic lipids.
- The two Gly-rich plasticins fold into well-defined helices in membrane environments.
- In micelles, Gly residues are located on the polar face of the amphipathic helix.
- GXXXG motifs in plasticins do not promote strong association between helices.
- The role of GXXXG motifs in plasticin could differ from that in transmembrane helices.
Plasticins (PTC) are dermaseptin-related antimicrobial peptides characterized by a large number of leucine and glycine residues arranged in GXXXG motifs that are often described to promote helix association within biological membranes. We report the structure and interaction properties of two plasticins, PTC-B1 from Phyllomedusa bicolor and a cationic analog of PTC-DA1 from Pachymedusa dacnicolor, which exhibit membrane-lytic activities on a broad range of microorganisms. Despite a high number of glycine, CD and NMR spectroscopy show that the two plasticins adopt mainly alpha-helical conformations in a wide variety of environments such as trifluoroethanol, detergent micelles and lipid vesicles. In DPC and SDS, plasticins adopt well-defined helices that lie parallel to the micelle surface, all glycine residues being located on the solvent-exposed face. Spectroscopic data and cross-linking experiments indicate that the GXXXG repeats in these amphipathic helices do not provide a strong oligomerization interface, suggesting a different role from GXXXG motifs found in transmembrane helices.
Journal: Biophysical Chemistry - Volume 196, January 2015, Pages 40-52