| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5370922 | 1503919 | 2015 | 8 صفحه PDF | دانلود رایگان |
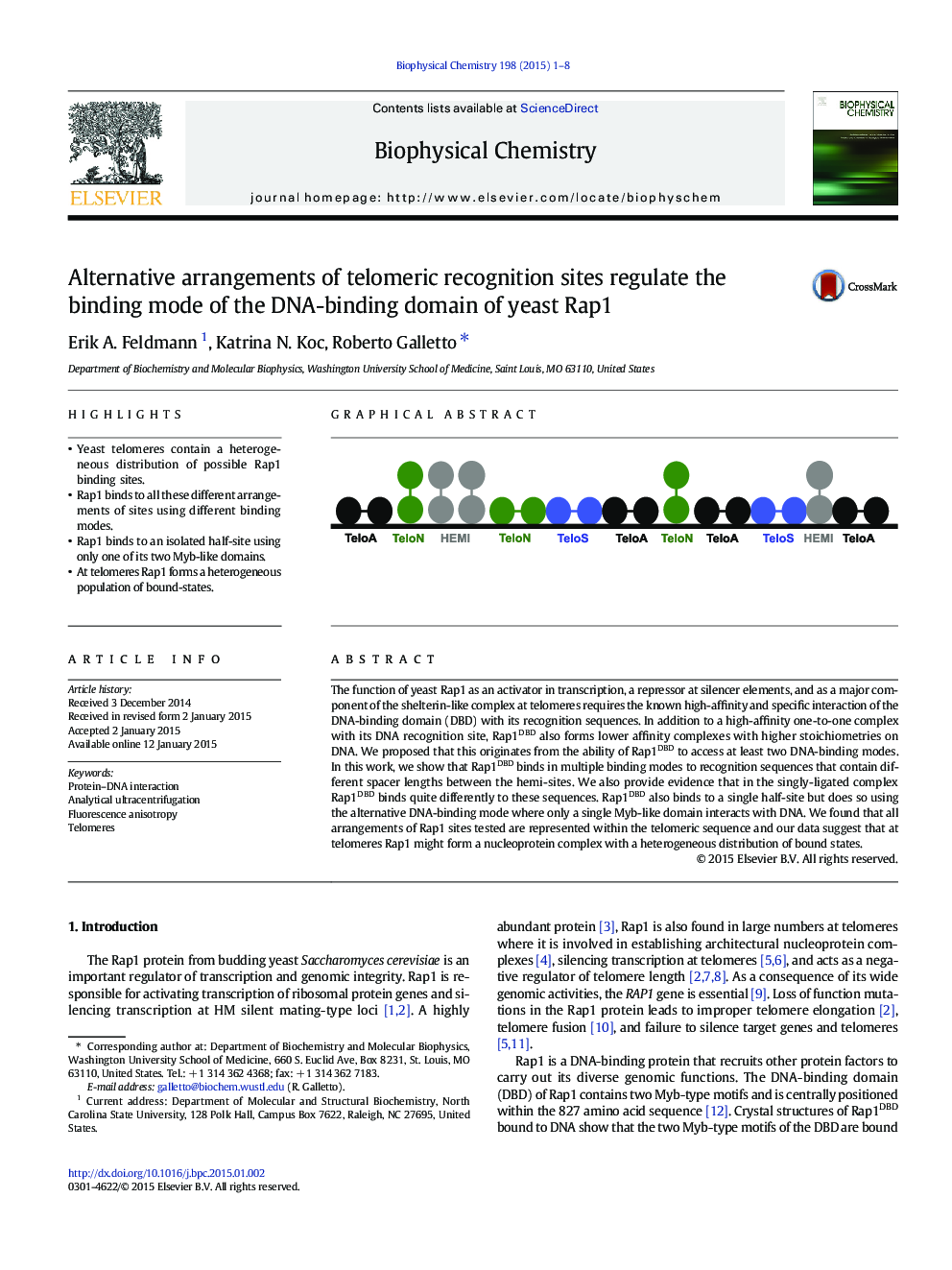
- Yeast telomeres contain a heterogeneous distribution of possible Rap1 binding sites.
- Rap1 binds to all these different arrangements of sites using different binding modes.
- Rap1 binds to an isolated half-site using only one of its two Myb-like domains.
- At telomeres Rap1 forms a heterogeneous population of bound-states.
The function of yeast Rap1 as an activator in transcription, a repressor at silencer elements, and as a major component of the shelterin-like complex at telomeres requires the known high-affinity and specific interaction of the DNA-binding domain (DBD) with its recognition sequences. In addition to a high-affinity one-to-one complex with its DNA recognition site, Rap1DBD also forms lower affinity complexes with higher stoichiometries on DNA. We proposed that this originates from the ability of Rap1DBD to access at least two DNA-binding modes. In this work, we show that Rap1DBD binds in multiple binding modes to recognition sequences that contain different spacer lengths between the hemi-sites. We also provide evidence that in the singly-ligated complex Rap1DBD binds quite differently to these sequences. Rap1DBD also binds to a single half-site but does so using the alternative DNA-binding mode where only a single Myb-like domain interacts with DNA. We found that all arrangements of Rap1 sites tested are represented within the telomeric sequence and our data suggest that at telomeres Rap1 might form a nucleoprotein complex with a heterogeneous distribution of bound states.
Journal: Biophysical Chemistry - Volume 198, March 2015, Pages 1-8