| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5371001 | 1503930 | 2013 | 10 صفحه PDF | دانلود رایگان |
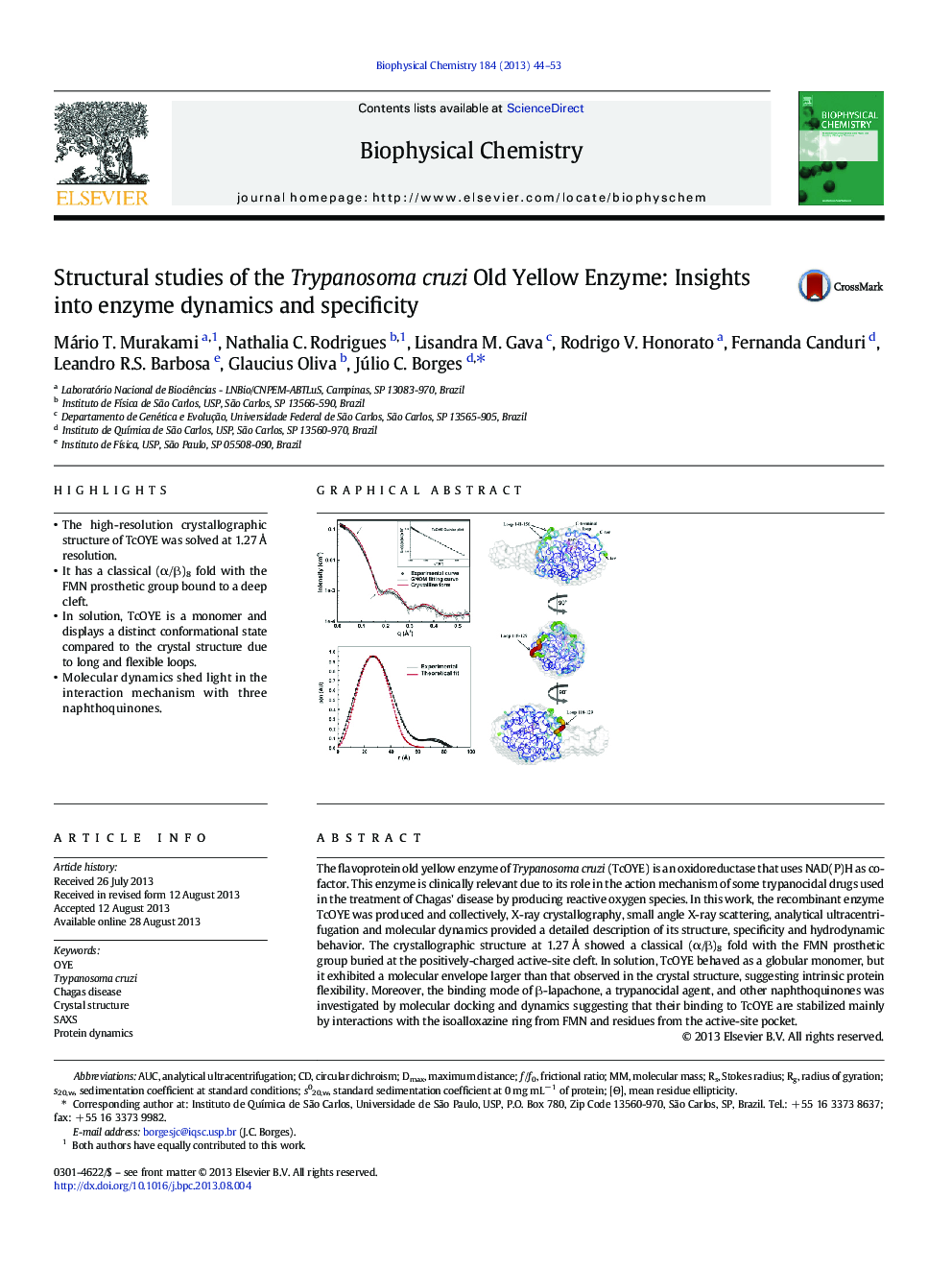
- The high-resolution crystallographic structure of TcOYE was solved at 1.27Â Ã
resolution.
- It has a classical (α/β)8 fold with the FMN prosthetic group bound to a deep cleft.
- In solution, TcOYE is a monomer and displays a distinct conformational state compared to the crystal structure due to long and flexible loops.
- Molecular dynamics shed light in the interaction mechanism with three naphthoquinones.
The flavoprotein old yellow enzyme of Trypanosoma cruzi (TcOYE) is an oxidoreductase that uses NAD(P)H as cofactor. This enzyme is clinically relevant due to its role in the action mechanism of some trypanocidal drugs used in the treatment of Chagas' disease by producing reactive oxygen species. In this work, the recombinant enzyme TcOYE was produced and collectively, X-ray crystallography, small angle X-ray scattering, analytical ultracentrifugation and molecular dynamics provided a detailed description of its structure, specificity and hydrodynamic behavior. The crystallographic structure at 1.27 à showed a classical (α/β)8 fold with the FMN prosthetic group buried at the positively-charged active-site cleft. In solution, TcOYE behaved as a globular monomer, but it exhibited a molecular envelope larger than that observed in the crystal structure, suggesting intrinsic protein flexibility. Moreover, the binding mode of β-lapachone, a trypanocidal agent, and other naphthoquinones was investigated by molecular docking and dynamics suggesting that their binding to TcOYE are stabilized mainly by interactions with the isoalloxazine ring from FMN and residues from the active-site pocket.
Journal: Biophysical Chemistry - Volume 184, 31 December 2013, Pages 44-53