| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5371202 | 1503941 | 2012 | 9 صفحه PDF | دانلود رایگان |
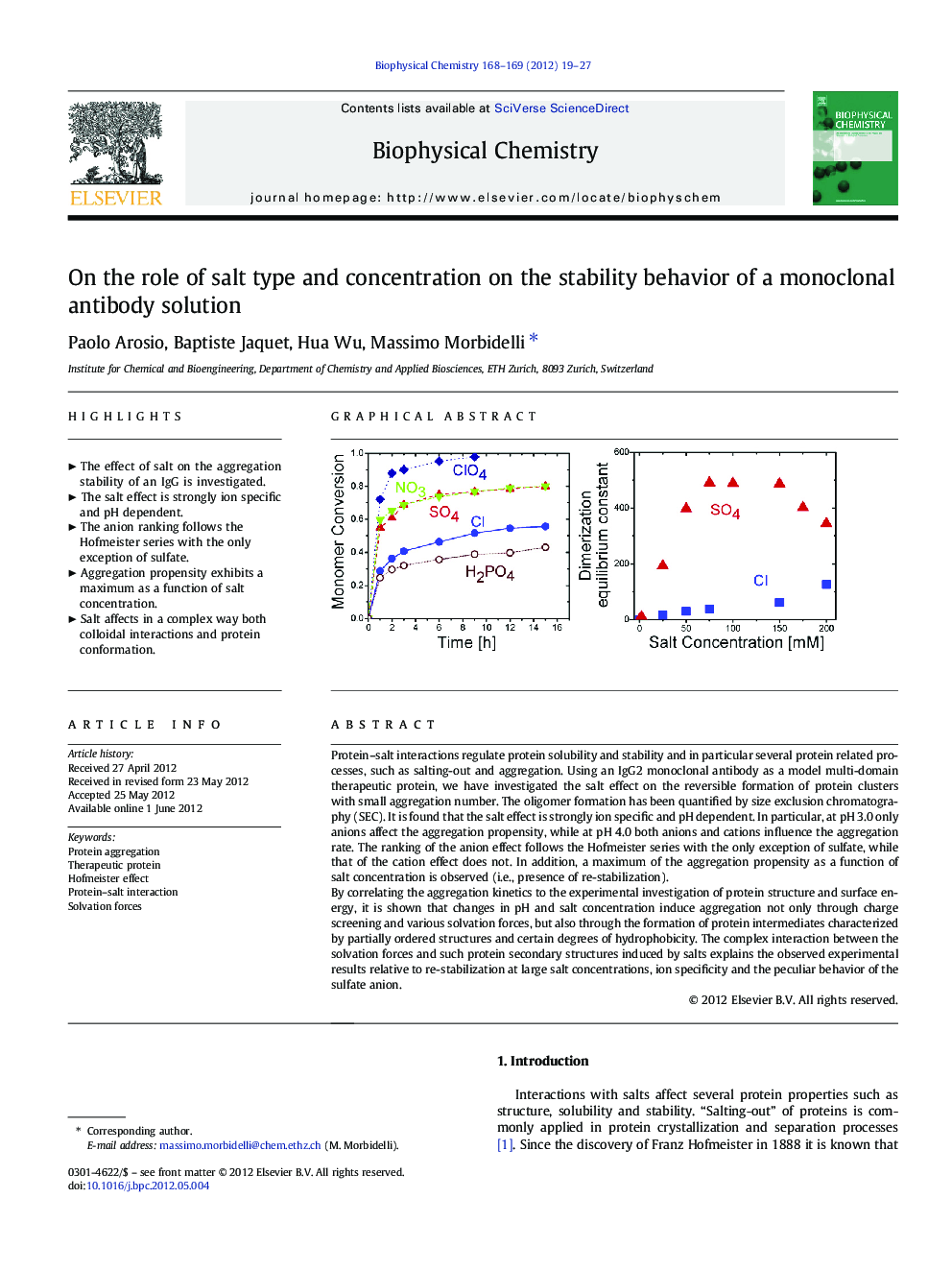
Protein-salt interactions regulate protein solubility and stability and in particular several protein related processes, such as salting-out and aggregation. Using an IgG2 monoclonal antibody as a model multi-domain therapeutic protein, we have investigated the salt effect on the reversible formation of protein clusters with small aggregation number. The oligomer formation has been quantified by size exclusion chromatography (SEC). It is found that the salt effect is strongly ion specific and pH dependent. In particular, at pH 3.0 only anions affect the aggregation propensity, while at pH 4.0 both anions and cations influence the aggregation rate. The ranking of the anion effect follows the Hofmeister series with the only exception of sulfate, while that of the cation effect does not. In addition, a maximum of the aggregation propensity as a function of salt concentration is observed (i.e., presence of re-stabilization).By correlating the aggregation kinetics to the experimental investigation of protein structure and surface energy, it is shown that changes in pH and salt concentration induce aggregation not only through charge screening and various solvation forces, but also through the formation of protein intermediates characterized by partially ordered structures and certain degrees of hydrophobicity. The complex interaction between the solvation forces and such protein secondary structures induced by salts explains the observed experimental results relative to re-stabilization at large salt concentrations, ion specificity and the peculiar behavior of the sulfate anion.
Highlights⺠The effect of salt on the aggregation stability of an IgG is investigated. ⺠The salt effect is strongly ion specific and pH dependent. ⺠The anion ranking follows the Hofmeister series with the only exception of sulfate. ⺠Aggregation propensity exhibits a maximum as a function of salt concentration. ⺠Salt affects in a complex way both colloidal interactions and protein conformation.
Journal: Biophysical Chemistry - Volumes 168â169, July 2012, Pages 19-27