| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5393301 | 1505567 | 2015 | 9 صفحه PDF | دانلود رایگان |
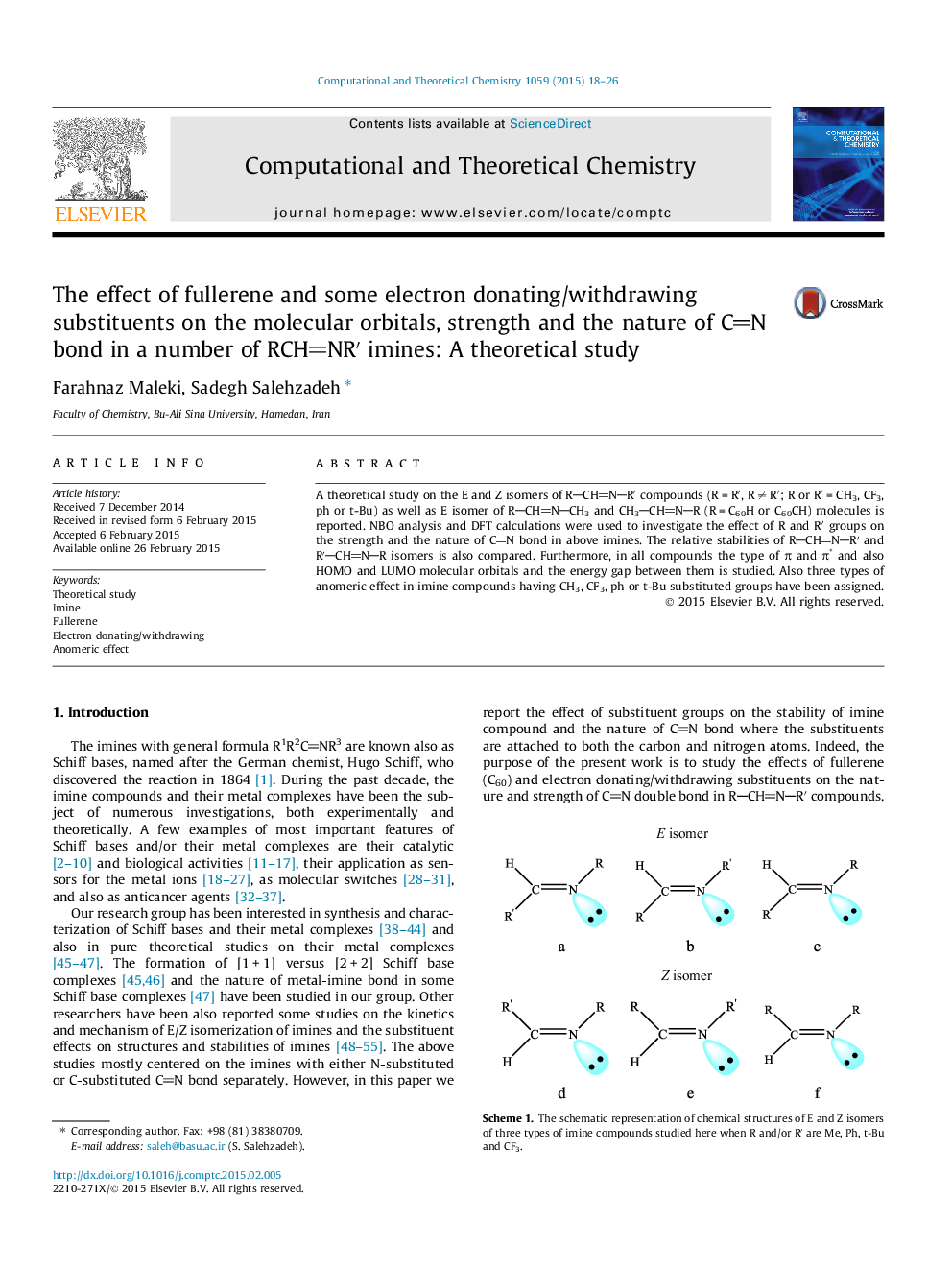
- E and Z isomers of RCHNRâ² compounds are studied.
- NBO analysis, interaction energies and relative stabilities are investigated.
- The type of molecular orbitals and the energy gap between them is studied.
- Electron withdrawing group on nitrogen atom increases the stability of imine.
- Fullerene substituent changes the type of frontier orbitals of imine molecules.
A theoretical study on the E and Z isomers of RCHNRâ² compounds (R = Râ², R â  Râ²; R or Râ²Â = CH3, CF3, ph or t-Bu) as well as E isomer of RCHNCH3 and CH3CHNR (R = C60H or C60CH) molecules is reported. NBO analysis and DFT calculations were used to investigate the effect of R and Râ² groups on the strength and the nature of CN bond in above imines. The relative stabilities of RCHNRâ² and Râ²CHNR isomers is also compared. Furthermore, in all compounds the type of Ï and Ï* and also HOMO and LUMO molecular orbitals and the energy gap between them is studied. Also three types of anomeric effect in imine compounds having CH3, CF3, ph or t-Bu substituted groups have been assigned.
Journal: Computational and Theoretical Chemistry - Volume 1059, 1 May 2015, Pages 18-26