| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5746015 | 1618785 | 2017 | 8 صفحه PDF | دانلود رایگان |
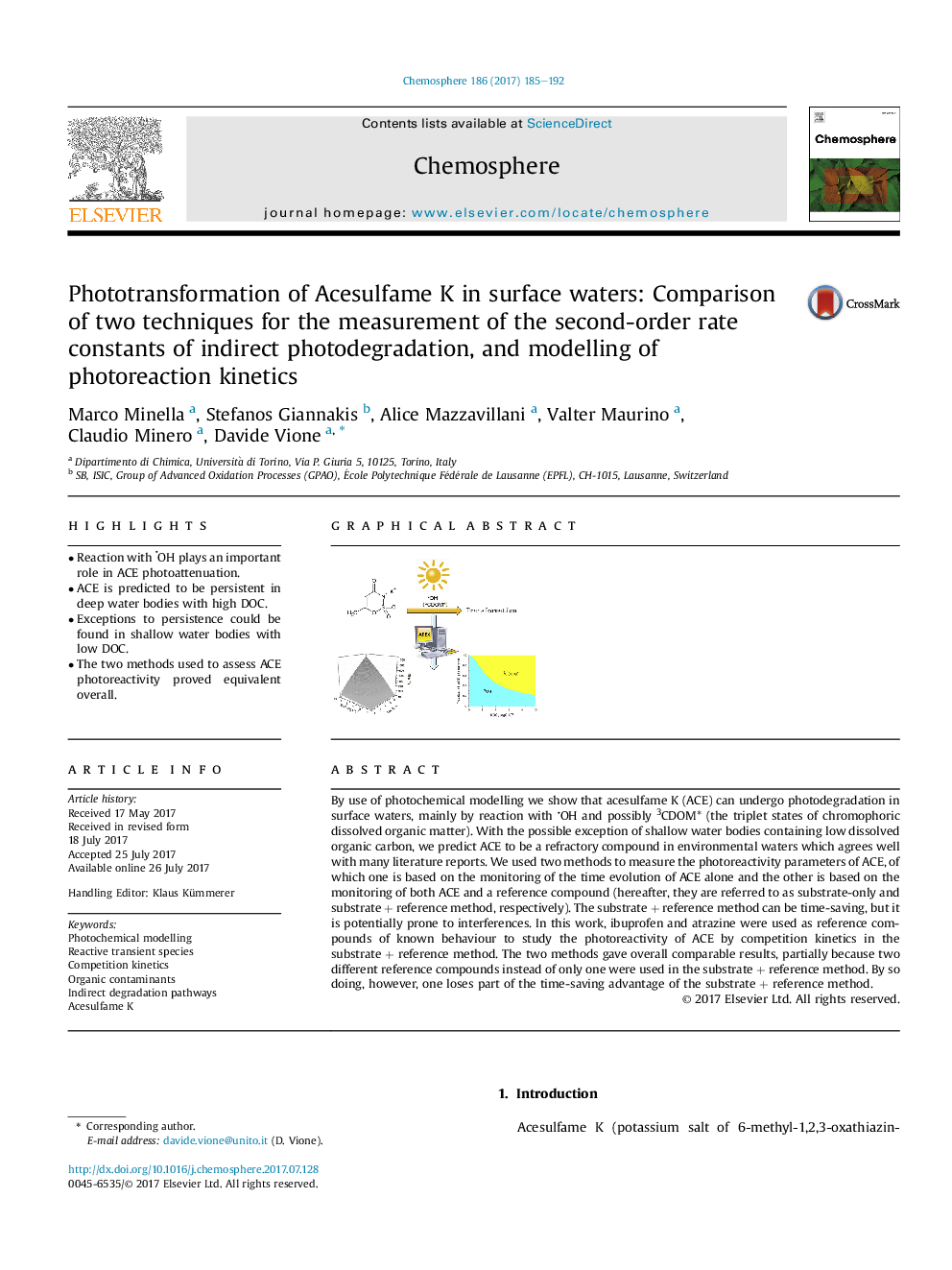
- Reaction with OH plays an important role in ACE photoattenuation.
- ACE is predicted to be persistent in deep water bodies with high DOC.
- Exceptions to persistence could be found in shallow water bodies with low DOC.
- The two methods used to assess ACE photoreactivity proved equivalent overall.
By use of photochemical modelling we show that acesulfame K (ACE) can undergo photodegradation in surface waters, mainly by reaction with OH and possibly 3CDOM* (the triplet states of chromophoric dissolved organic matter). With the possible exception of shallow water bodies containing low dissolved organic carbon, we predict ACE to be a refractory compound in environmental waters which agrees well with many literature reports. We used two methods to measure the photoreactivity parameters of ACE, of which one is based on the monitoring of the time evolution of ACE alone and the other is based on the monitoring of both ACE and a reference compound (hereafter, they are referred to as substrate-only and substrate + reference method, respectively). The substrate + reference method can be time-saving, but it is potentially prone to interferences. In this work, ibuprofen and atrazine were used as reference compounds of known behaviour to study the photoreactivity of ACE by competition kinetics in the substrate + reference method. The two methods gave overall comparable results, partially because two different reference compounds instead of only one were used in the substrate + reference method. By so doing, however, one loses part of the time-saving advantage of the substrate + reference method.
132
Journal: Chemosphere - Volume 186, November 2017, Pages 185-192