| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5746233 | 1618787 | 2017 | 6 صفحه PDF | دانلود رایگان |
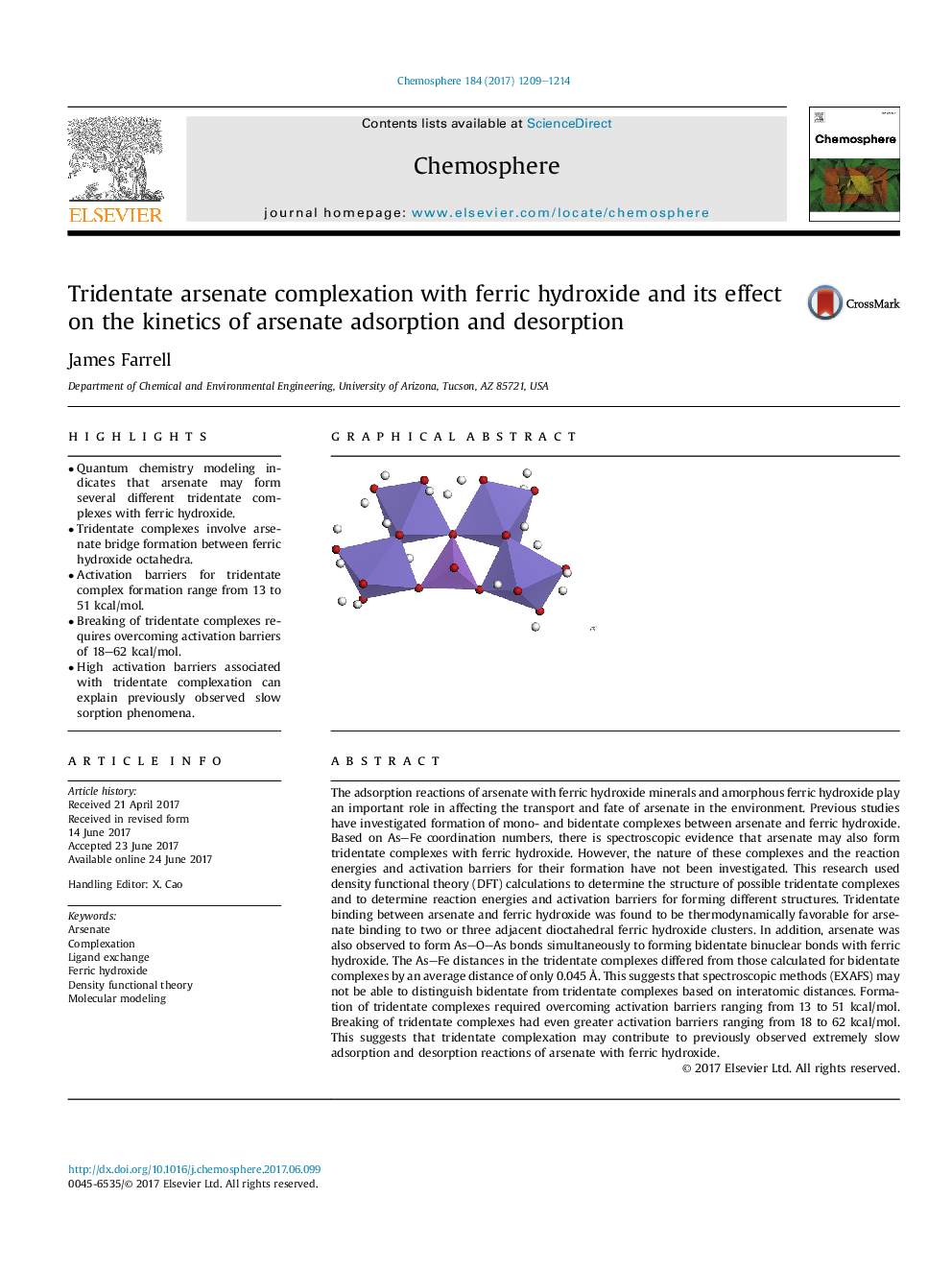
- Quantum chemistry modeling indicates that arsenate may form several different tridentate complexes with ferric hydroxide.
- Tridentate complexes involve arsenate bridge formation between ferric hydroxide octahedra.
- Activation barriers for tridentate complex formation range from 13 to 51 kcal/mol.
- Breaking of tridentate complexes requires overcoming activation barriers of 18-62Â kcal/mol.
- High activation barriers associated with tridentate complexation can explain previously observed slow sorption phenomena.
The adsorption reactions of arsenate with ferric hydroxide minerals and amorphous ferric hydroxide play an important role in affecting the transport and fate of arsenate in the environment. Previous studies have investigated formation of mono- and bidentate complexes between arsenate and ferric hydroxide. Based on AsFe coordination numbers, there is spectroscopic evidence that arsenate may also form tridentate complexes with ferric hydroxide. However, the nature of these complexes and the reaction energies and activation barriers for their formation have not been investigated. This research used density functional theory (DFT) calculations to determine the structure of possible tridentate complexes and to determine reaction energies and activation barriers for forming different structures. Tridentate binding between arsenate and ferric hydroxide was found to be thermodynamically favorable for arsenate binding to two or three adjacent dioctahedral ferric hydroxide clusters. In addition, arsenate was also observed to form AsOAs bonds simultaneously to forming bidentate binuclear bonds with ferric hydroxide. The AsFe distances in the tridentate complexes differed from those calculated for bidentate complexes by an average distance of only 0.045Â Ã . This suggests that spectroscopic methods (EXAFS) may not be able to distinguish bidentate from tridentate complexes based on interatomic distances. Formation of tridentate complexes required overcoming activation barriers ranging from 13 to 51Â kcal/mol. Breaking of tridentate complexes had even greater activation barriers ranging from 18 to 62Â kcal/mol. This suggests that tridentate complexation may contribute to previously observed extremely slow adsorption and desorption reactions of arsenate with ferric hydroxide.
133
Journal: Chemosphere - Volume 184, October 2017, Pages 1209-1214