| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 591571 | 1453873 | 2016 | 9 صفحه PDF | دانلود رایگان |
• A novel and low-cost nanocomposite was prepared and used for Cu(II) ions removal.
• The adsorption of the nanocomposite for Cu(II) ions has strong pH-dependence.
• The effect of pH value of solution on adsorption process and mechanism was studied.
• XPS and FTIR measurements were combined to analyze adsorption mechanisms.
• This work will contribute to design more efficient materials for Cu(II) adsorption.
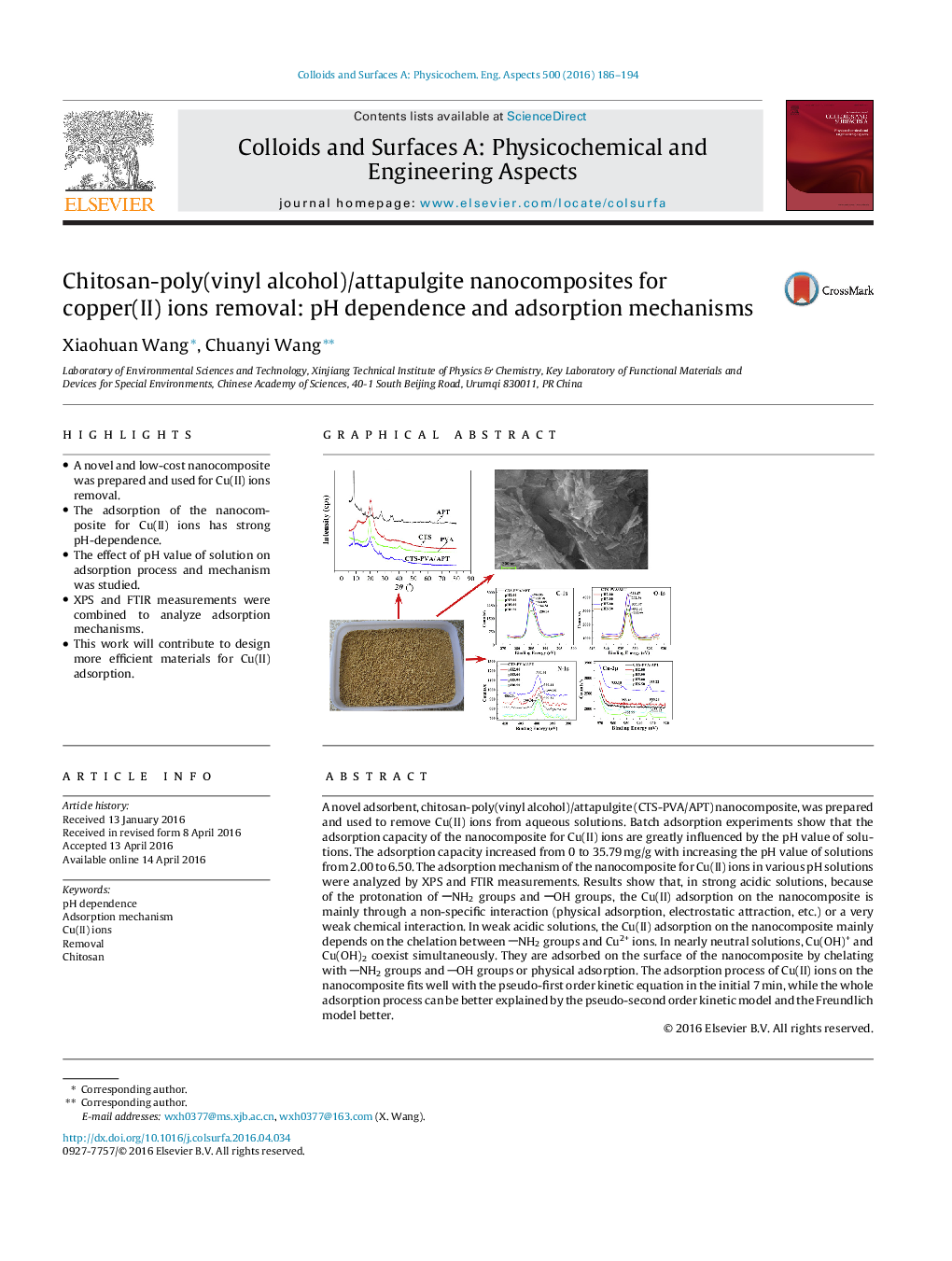
A novel adsorbent, chitosan-poly(vinyl alcohol)/attapulgite (CTS-PVA/APT) nanocomposite, was prepared and used to remove Cu(II) ions from aqueous solutions. Batch adsorption experiments show that the adsorption capacity of the nanocomposite for Cu(II) ions are greatly influenced by the pH value of solutions. The adsorption capacity increased from 0 to 35.79 mg/g with increasing the pH value of solutions from 2.00 to 6.50. The adsorption mechanism of the nanocomposite for Cu(II) ions in various pH solutions were analyzed by XPS and FTIR measurements. Results show that, in strong acidic solutions, because of the protonation of NH2 groups and OH groups, the Cu(II) adsorption on the nanocomposite is mainly through a non-specific interaction (physical adsorption, electrostatic attraction, etc.) or a very weak chemical interaction. In weak acidic solutions, the Cu(II) adsorption on the nanocomposite mainly depends on the chelation between NH2 groups and Cu2+ ions. In nearly neutral solutions, Cu(OH)+ and Cu(OH)2 coexist simultaneously. They are adsorbed on the surface of the nanocomposite by chelating with NH2 groups and OH groups or physical adsorption. The adsorption process of Cu(II) ions on the nanocomposite fits well with the pseudo-first order kinetic equation in the initial 7 min, while the whole adsorption process can be better explained by the pseudo-second order kinetic model and the Freundlich model better.
Figure optionsDownload as PowerPoint slide
Journal: Colloids and Surfaces A: Physicochemical and Engineering Aspects - Volume 500, 5 July 2016, Pages 186–194