| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 591586 | 1453871 | 2016 | 8 صفحه PDF | دانلود رایگان |
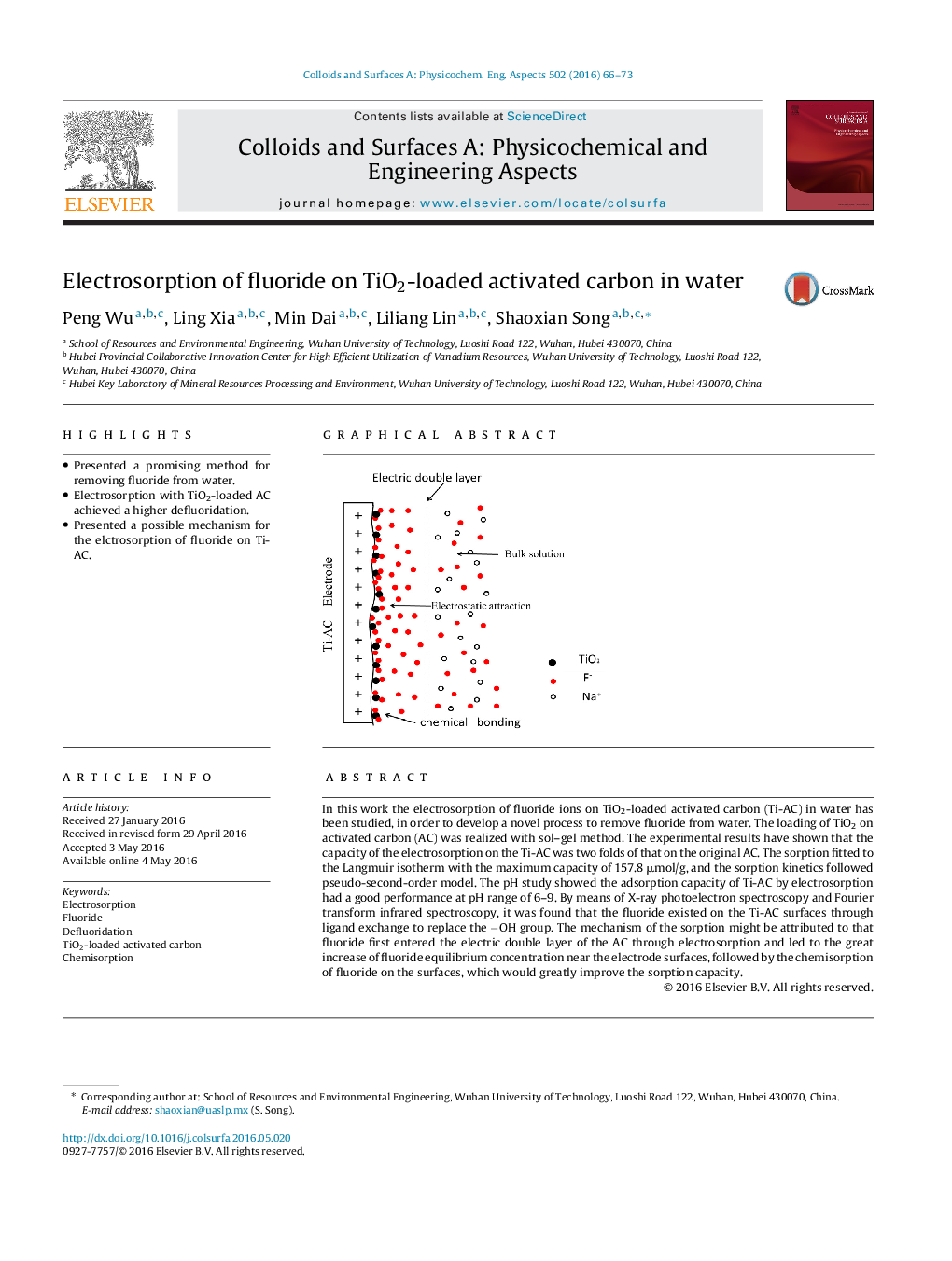
• Presented a promising method for removing fluoride from water.
• Electrosorption with TiO2-loaded AC achieved a higher defluoridation.
• Presented a possible mechanism for the elctrosorption of fluoride on Ti-AC.
In this work the electrosorption of fluoride ions on TiO2-loaded activated carbon (Ti-AC) in water has been studied, in order to develop a novel process to remove fluoride from water. The loading of TiO2 on activated carbon (AC) was realized with sol–gel method. The experimental results have shown that the capacity of the electrosorption on the Ti-AC was two folds of that on the original AC. The sorption fitted to the Langmuir isotherm with the maximum capacity of 157.8 μmol/g, and the sorption kinetics followed pseudo-second-order model. The pH study showed the adsorption capacity of Ti-AC by electrosorption had a good performance at pH range of 6–9. By means of X-ray photoelectron spectroscopy and Fourier transform infrared spectroscopy, it was found that the fluoride existed on the Ti-AC surfaces through ligand exchange to replace the −OH group. The mechanism of the sorption might be attributed to that fluoride first entered the electric double layer of the AC through electrosorption and led to the great increase of fluoride equilibrium concentration near the electrode surfaces, followed by the chemisorption of fluoride on the surfaces, which would greatly improve the sorption capacity.
Figure optionsDownload as PowerPoint slide
Journal: Colloids and Surfaces A: Physicochemical and Engineering Aspects - Volume 502, 5 August 2016, Pages 66–73