| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 591801 | 1453883 | 2016 | 8 صفحه PDF | دانلود رایگان |
• A hydrophobic associating cationic polyacrylamide with N,N′-dimethyl octadeyl allyl ammonium chloride was synthesized.
• This hydrophobic associating cationic polyacrylamide shows an unusual pH-responsive sol–gel transition.
• Mechanism analysis has confirmed that this pH-responsive behavior is attribute to counter ions effect and hydrogen bond.
• We provide a new path to fabricate smart hydrogel and provides a novel thinking to interpret the similar phenomenon.
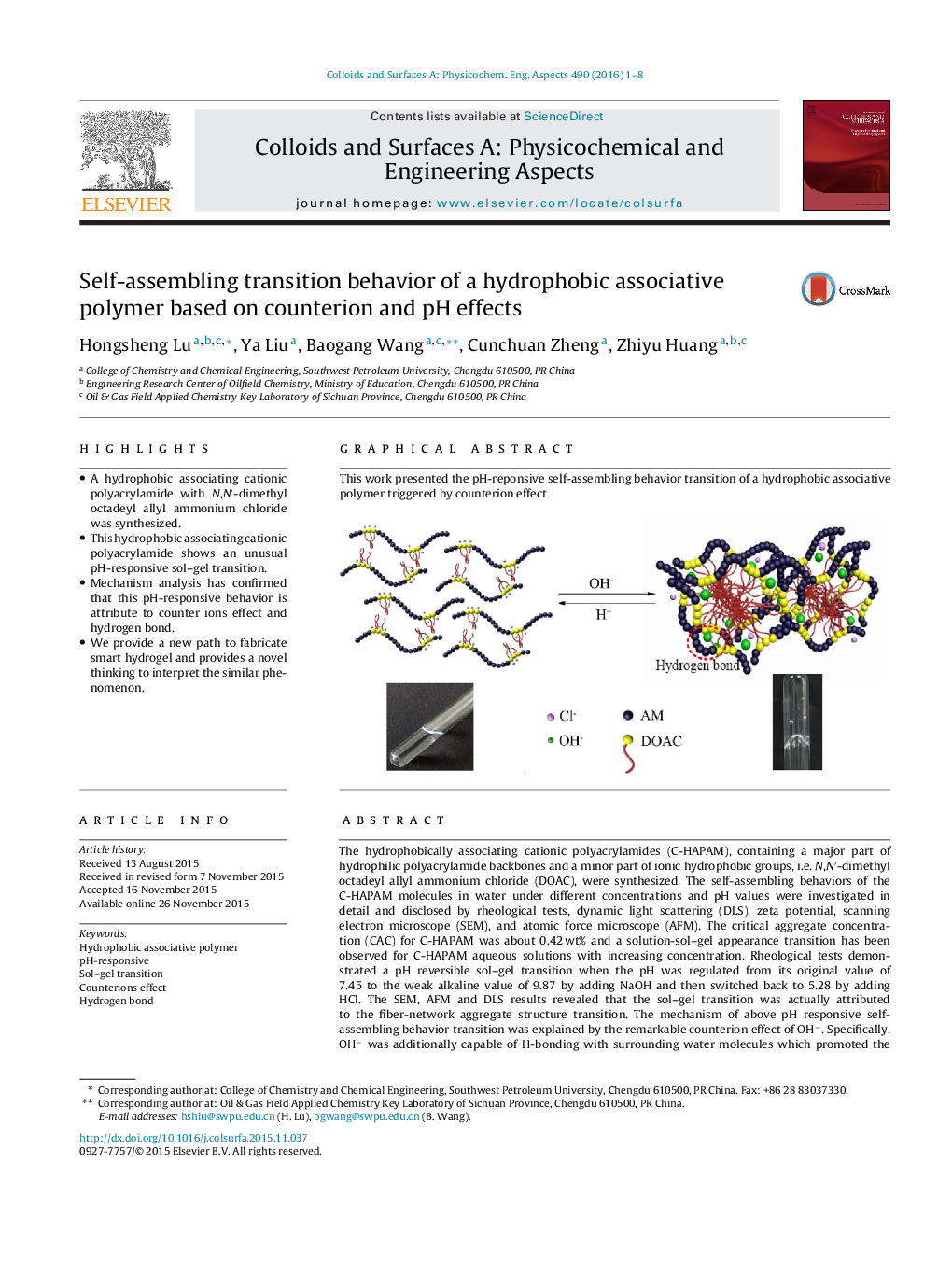
The hydrophobically associating cationic polyacrylamides (C-HAPAM), containing a major part of hydrophilic polyacrylamide backbones and a minor part of ionic hydrophobic groups, i.e. N,N′-dimethyl octadeyl allyl ammonium chloride (DOAC), were synthesized. The self-assembling behaviors of the C-HAPAM molecules in water under different concentrations and pH values were investigated in detail and disclosed by rheological tests, dynamic light scattering (DLS), zeta potential, scanning electron microscope (SEM), and atomic force microscope (AFM). The critical aggregate concentration (CAC) for C-HAPAM was about 0.42 wt% and a solution-sol–gel appearance transition has been observed for C-HAPAM aqueous solutions with increasing concentration. Rheological tests demonstrated a pH reversible sol–gel transition when the pH was regulated from its original value of 7.45 to the weak alkaline value of 9.87 by adding NaOH and then switched back to 5.28 by adding HCl. The SEM, AFM and DLS results revealed that the sol–gel transition was actually attributed to the fiber-network aggregate structure transition. The mechanism of above pH responsive self-assembling behavior transition was explained by the remarkable counterion effect of OH−. Specifically, OH− was additionally capable of H-bonding with surrounding water molecules which promoted the solvation of C-HAPAM and induced thick hydration layer. Then, the hydrated layer led to the increment of zeta potential and volume expansion of C-HAPAM molecule which in turn strongly enhanced the viscosity of the solution. The strong counterion effect of OH− and the inter-molecular hydrophobic association interactions of C-HAPAM collectively triggered the formation of joints and hence the network structure transition, i.e. sol–gel transition.
This work presented the pH-reponsive self-assembling behavior transition of a hydrophobic associative polymer triggered by counterion effectFigure optionsDownload as PowerPoint slide
Journal: Colloids and Surfaces A: Physicochemical and Engineering Aspects - Volume 490, 5 February 2016, Pages 1–8