| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 591898 | 1453884 | 2016 | 11 صفحه PDF | دانلود رایگان |
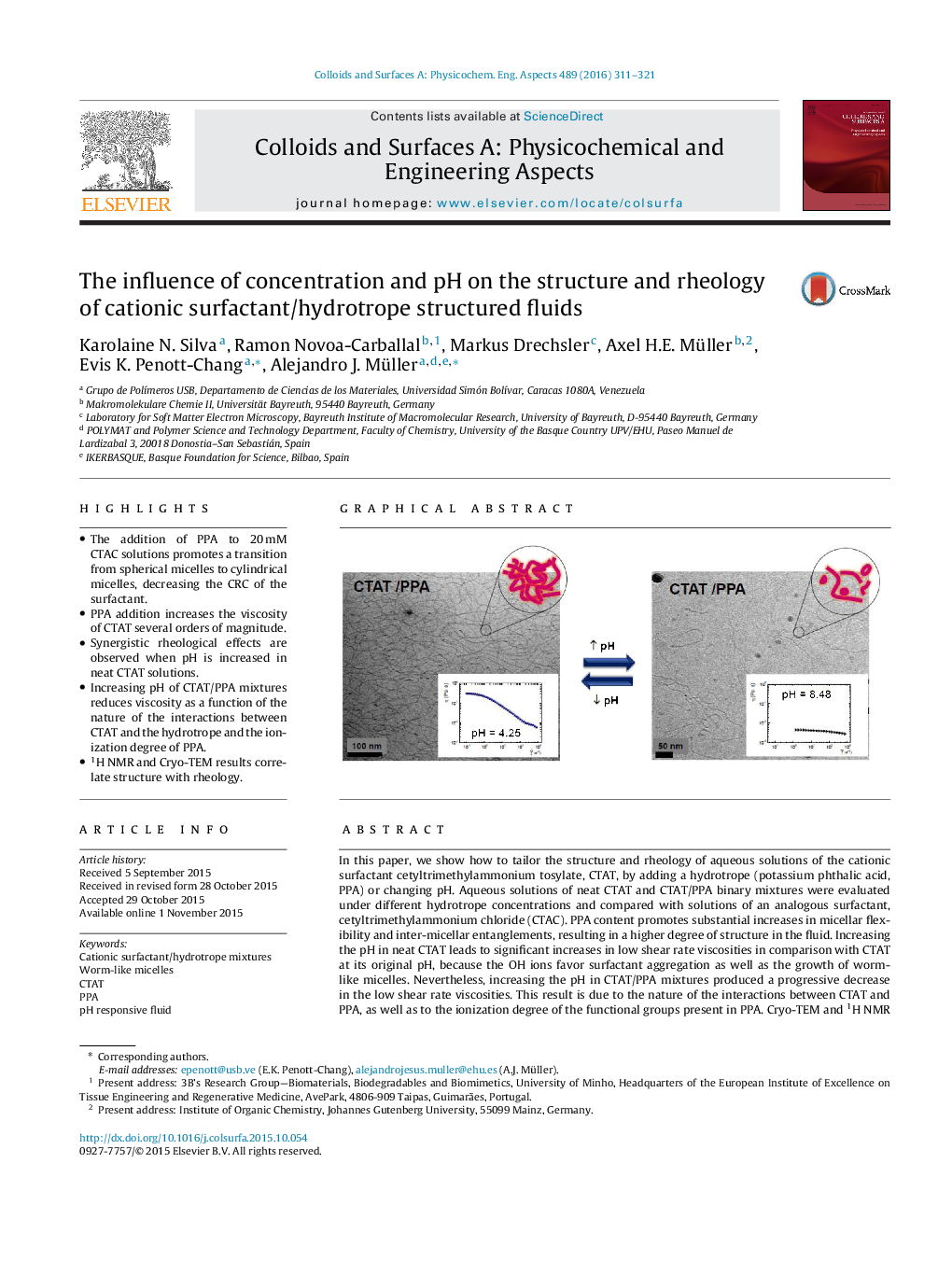
• The addition of PPA to 20 mM CTAC solutions promotes a transition from spherical micelles to cylindrical micelles, decreasing the CRC of the surfactant.
• PPA addition increases the viscosity of CTAT several orders of magnitude.
• Synergistic rheological effects are observed when pH is increased in neat CTAT solutions.
• Increasing pH of CTAT/PPA mixtures reduces viscosity as a function of the nature of the interactions between CTAT and the hydrotrope and the ionization degree of PPA.
• 1H NMR and Cryo-TEM results correlate structure with rheology.
In this paper, we show how to tailor the structure and rheology of aqueous solutions of the cationic surfactant cetyltrimethylammonium tosylate, CTAT, by adding a hydrotrope (potassium phthalic acid, PPA) or changing pH. Aqueous solutions of neat CTAT and CTAT/PPA binary mixtures were evaluated under different hydrotrope concentrations and compared with solutions of an analogous surfactant, cetyltrimethylammonium chloride (CTAC). PPA content promotes substantial increases in micellar flexibility and inter-micellar entanglements, resulting in a higher degree of structure in the fluid. Increasing the pH in neat CTAT leads to significant increases in low shear rate viscosities in comparison with CTAT at its original pH, because the OH ions favor surfactant aggregation as well as the growth of worm-like micelles. Nevertheless, increasing the pH in CTAT/PPA mixtures produced a progressive decrease in the low shear rate viscosities. This result is due to the nature of the interactions between CTAT and PPA, as well as to the ionization degree of the functional groups present in PPA. Cryo-TEM and 1H NMR measurements evidenced, respectively, the microstructural and chemical environmental changes of CTAT upon PPA addition and explain the rheological properties of the CTAT/PPA mixtures as a function of the fluid structure.
Figure optionsDownload as PowerPoint slide
Journal: Colloids and Surfaces A: Physicochemical and Engineering Aspects - Volume 489, 20 January 2016, Pages 311–321