| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 592616 | 1453912 | 2014 | 13 صفحه PDF | دانلود رایگان |
• Graphite deposits are formed by evaporation of graphite/SDS dispersion drops.
• Uniform spherical cap deposits are obtained on hydrophobic Teflon and FS surfaces.
• Deposit diameters and heights vary linearly with surface free energy of substrates.
• Graphite and SDS concentrations affect the diameter and heights of stains.
• 3D size control of graphite deposits is successfully obtained.
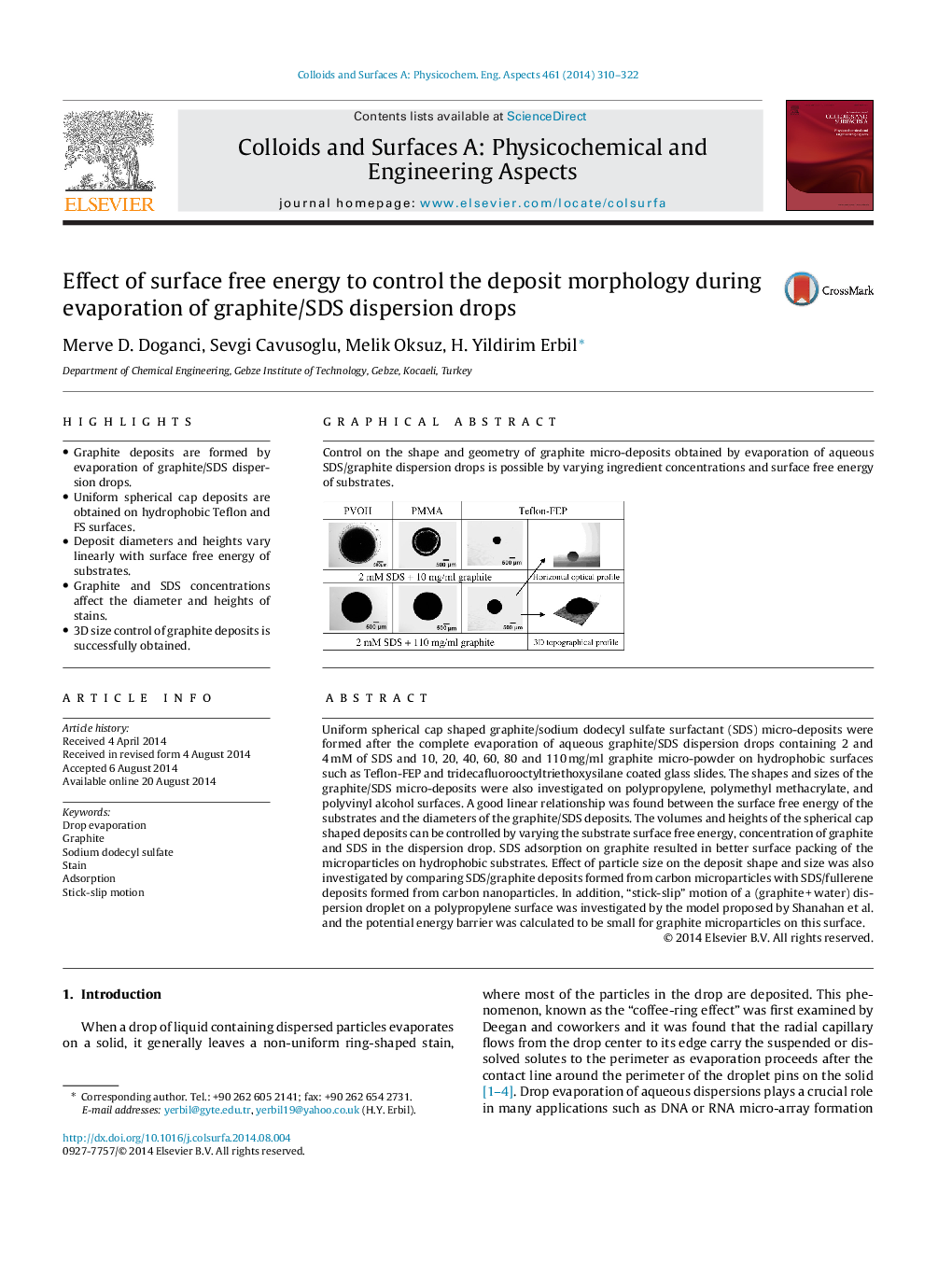
Uniform spherical cap shaped graphite/sodium dodecyl sulfate surfactant (SDS) micro-deposits were formed after the complete evaporation of aqueous graphite/SDS dispersion drops containing 2 and 4 mM of SDS and 10, 20, 40, 60, 80 and 110 mg/ml graphite micro-powder on hydrophobic surfaces such as Teflon-FEP and tridecafluorooctyltriethoxysilane coated glass slides. The shapes and sizes of the graphite/SDS micro-deposits were also investigated on polypropylene, polymethyl methacrylate, and polyvinyl alcohol surfaces. A good linear relationship was found between the surface free energy of the substrates and the diameters of the graphite/SDS deposits. The volumes and heights of the spherical cap shaped deposits can be controlled by varying the substrate surface free energy, concentration of graphite and SDS in the dispersion drop. SDS adsorption on graphite resulted in better surface packing of the microparticles on hydrophobic substrates. Effect of particle size on the deposit shape and size was also investigated by comparing SDS/graphite deposits formed from carbon microparticles with SDS/fullerene deposits formed from carbon nanoparticles. In addition, “stick-slip” motion of a (graphite + water) dispersion droplet on a polypropylene surface was investigated by the model proposed by Shanahan et al. and the potential energy barrier was calculated to be small for graphite microparticles on this surface.
Control on the shape and geometry of graphite micro-deposits obtained by evaporation of aqueous SDS/graphite dispersion drops is possible by varying ingredient concentrations and surface free energy of substrates.Figure optionsDownload as PowerPoint slide
Journal: Colloids and Surfaces A: Physicochemical and Engineering Aspects - Volume 461, 5 November 2014, Pages 310–322