| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 593637 | 1453947 | 2013 | 8 صفحه PDF | دانلود رایگان |
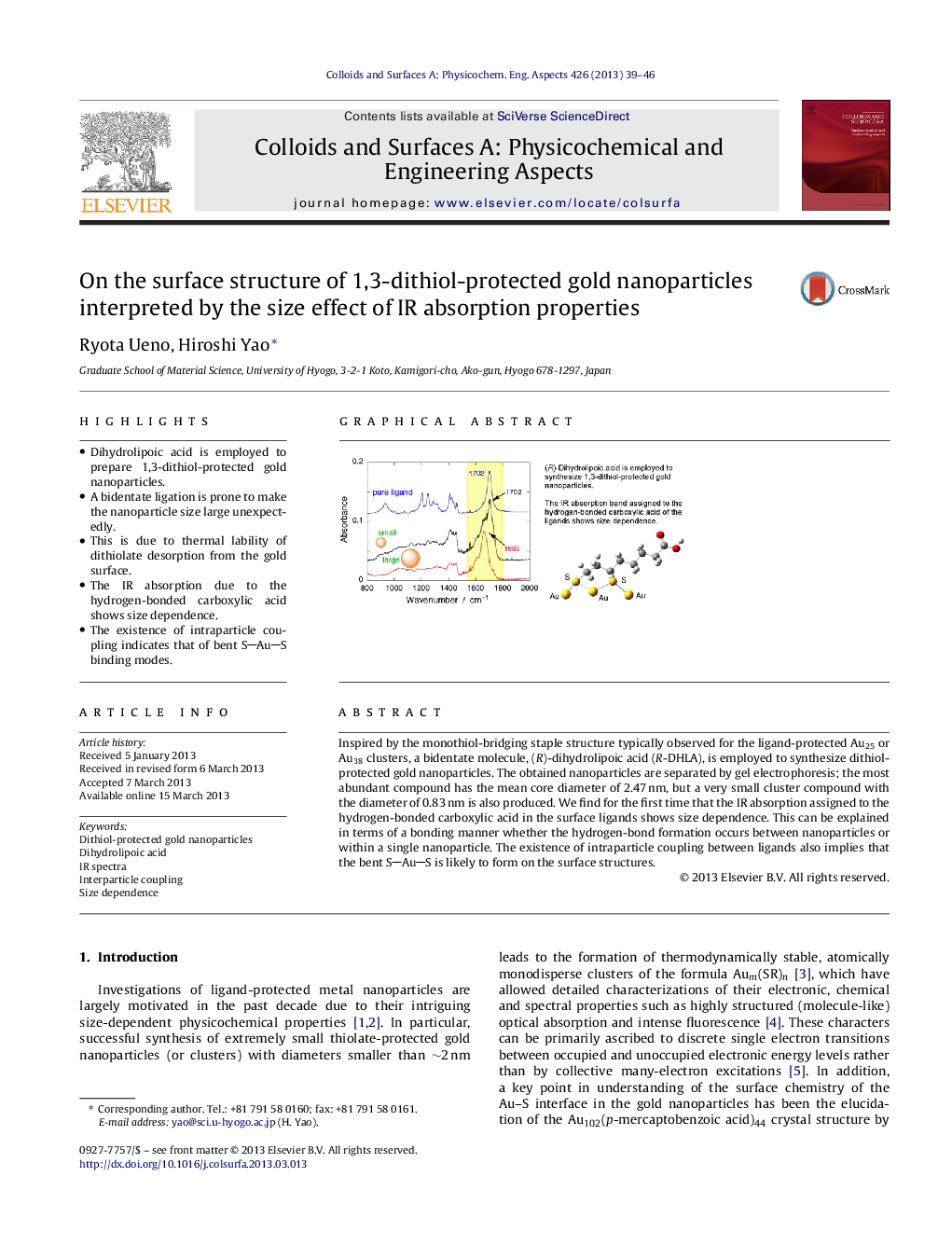
• Dihydrolipoic acid is employed to prepare 1,3-dithiol-protected gold nanoparticles.
• A bidentate ligation is prone to make the nanoparticle size large unexpectedly.
• This is due to thermal lability of dithiolate desorption from the gold surface.
• The IR absorption due to the hydrogen-bonded carboxylic acid shows size dependence.
• The existence of intraparticle coupling indicates that of bent SAuS binding modes.
Inspired by the monothiol-bridging staple structure typically observed for the ligand-protected Au25 or Au38 clusters, a bidentate molecule, (R)-dihydrolipoic acid (R-DHLA), is employed to synthesize dithiol-protected gold nanoparticles. The obtained nanoparticles are separated by gel electrophoresis; the most abundant compound has the mean core diameter of 2.47 nm, but a very small cluster compound with the diameter of 0.83 nm is also produced. We find for the first time that the IR absorption assigned to the hydrogen-bonded carboxylic acid in the surface ligands shows size dependence. This can be explained in terms of a bonding manner whether the hydrogen-bond formation occurs between nanoparticles or within a single nanoparticle. The existence of intraparticle coupling between ligands also implies that the bent SAuS is likely to form on the surface structures.
Figure optionsDownload as PowerPoint slide
Journal: Colloids and Surfaces A: Physicochemical and Engineering Aspects - Volume 426, 5 June 2013, Pages 39–46