| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 593762 | 1453954 | 2013 | 7 صفحه PDF | دانلود رایگان |
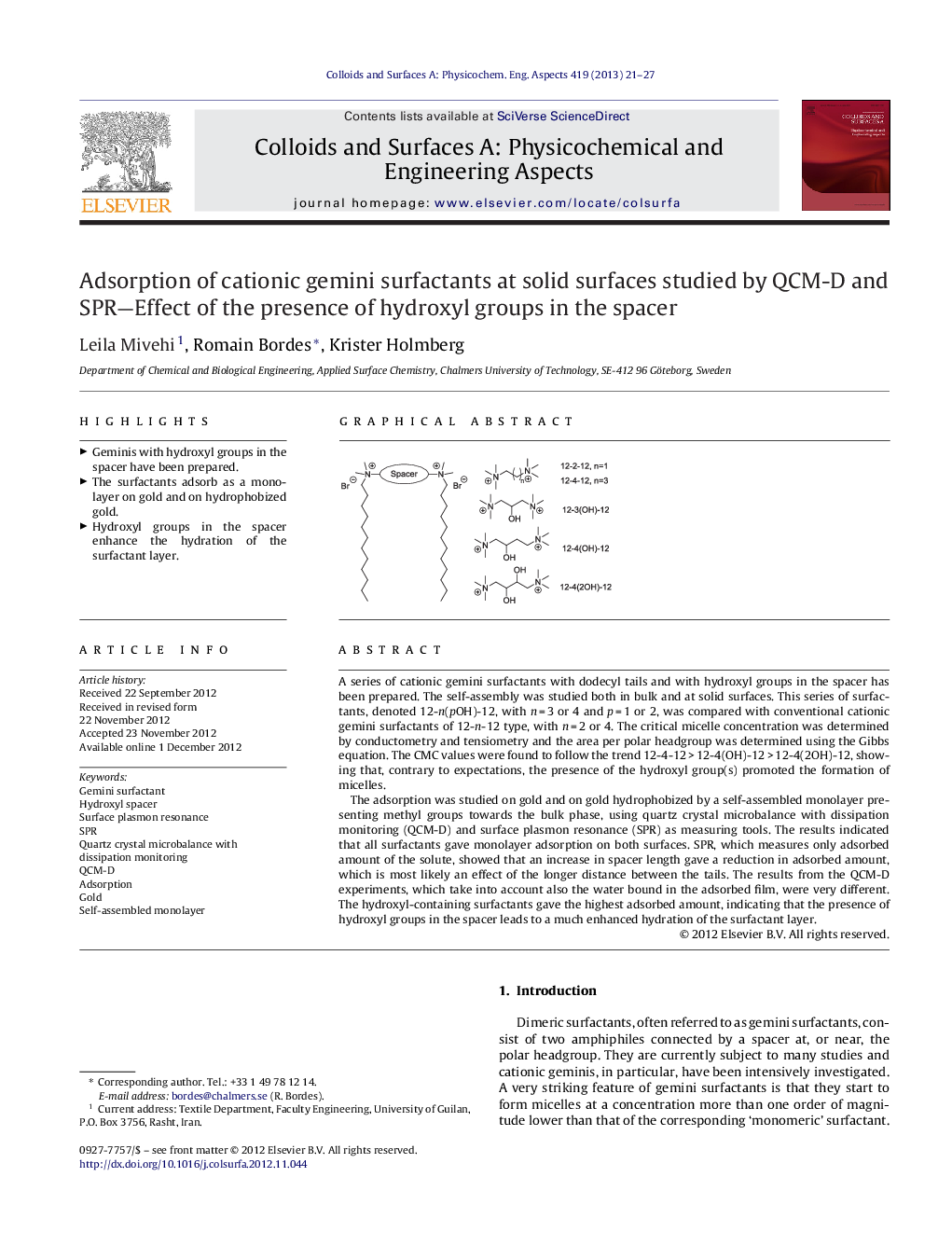
A series of cationic gemini surfactants with dodecyl tails and with hydroxyl groups in the spacer has been prepared. The self-assembly was studied both in bulk and at solid surfaces. This series of surfactants, denoted 12-n(pOH)-12, with n = 3 or 4 and p = 1 or 2, was compared with conventional cationic gemini surfactants of 12-n-12 type, with n = 2 or 4. The critical micelle concentration was determined by conductometry and tensiometry and the area per polar headgroup was determined using the Gibbs equation. The CMC values were found to follow the trend 12-4-12 > 12-4(OH)-12 > 12-4(2OH)-12, showing that, contrary to expectations, the presence of the hydroxyl group(s) promoted the formation of micelles.The adsorption was studied on gold and on gold hydrophobized by a self-assembled monolayer presenting methyl groups towards the bulk phase, using quartz crystal microbalance with dissipation monitoring (QCM-D) and surface plasmon resonance (SPR) as measuring tools. The results indicated that all surfactants gave monolayer adsorption on both surfaces. SPR, which measures only adsorbed amount of the solute, showed that an increase in spacer length gave a reduction in adsorbed amount, which is most likely an effect of the longer distance between the tails. The results from the QCM-D experiments, which take into account also the water bound in the adsorbed film, were very different. The hydroxyl-containing surfactants gave the highest adsorbed amount, indicating that the presence of hydroxyl groups in the spacer leads to a much enhanced hydration of the surfactant layer.
Figure optionsDownload as PowerPoint slideHighlights
► Geminis with hydroxyl groups in the spacer have been prepared.
► The surfactants adsorb as a monolayer on gold and on hydrophobized gold.
► Hydroxyl groups in the spacer enhance the hydration of the surfactant layer.
Journal: Colloids and Surfaces A: Physicochemical and Engineering Aspects - Volume 419, 20 February 2013, Pages 21–27