| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 593768 | 1453954 | 2013 | 11 صفحه PDF | دانلود رایگان |
Multi-walled carbon nanotubes/manganese oxide (MWCNTs/MnO2) nanocomposite was synthesized, characterized, and used successfully for the removal of lead ions from an aqueous solution and waste water sample. The characterization techniques showed that MWCNTs were coated with 10.2% of the crystalline α-MnO2 (w/w percent). The adsorption process was optimized, and the results showed that most of the lead could be removed from a solution with 10 mg MWCNTs/MnO2 nanocomposite, at a pH between 7.0 and 9.0, and within a few min. The adsorption was studied kinetically, and the results revealed that the adsorption of lead ions by a MWCNTs/MnO2 nanocomposite from an aqueous solution could be described well by a pseudo-second-order model and the Elovich model. The mechanism of adsorption showed that the adsorption process was complex and involves a different step, but it was mainly controlled by a liquid film diffusion mechanism. The thermodynamic parameters were calculated, and the adsorption was found to be chemical, spontaneous, and endothermic in nature with positive entropy. The MWCNTs/MnO2 nanocomposite was used for the removal of lead ions from a spiked waste water sample, and the results showed that all the lead ions were removed.
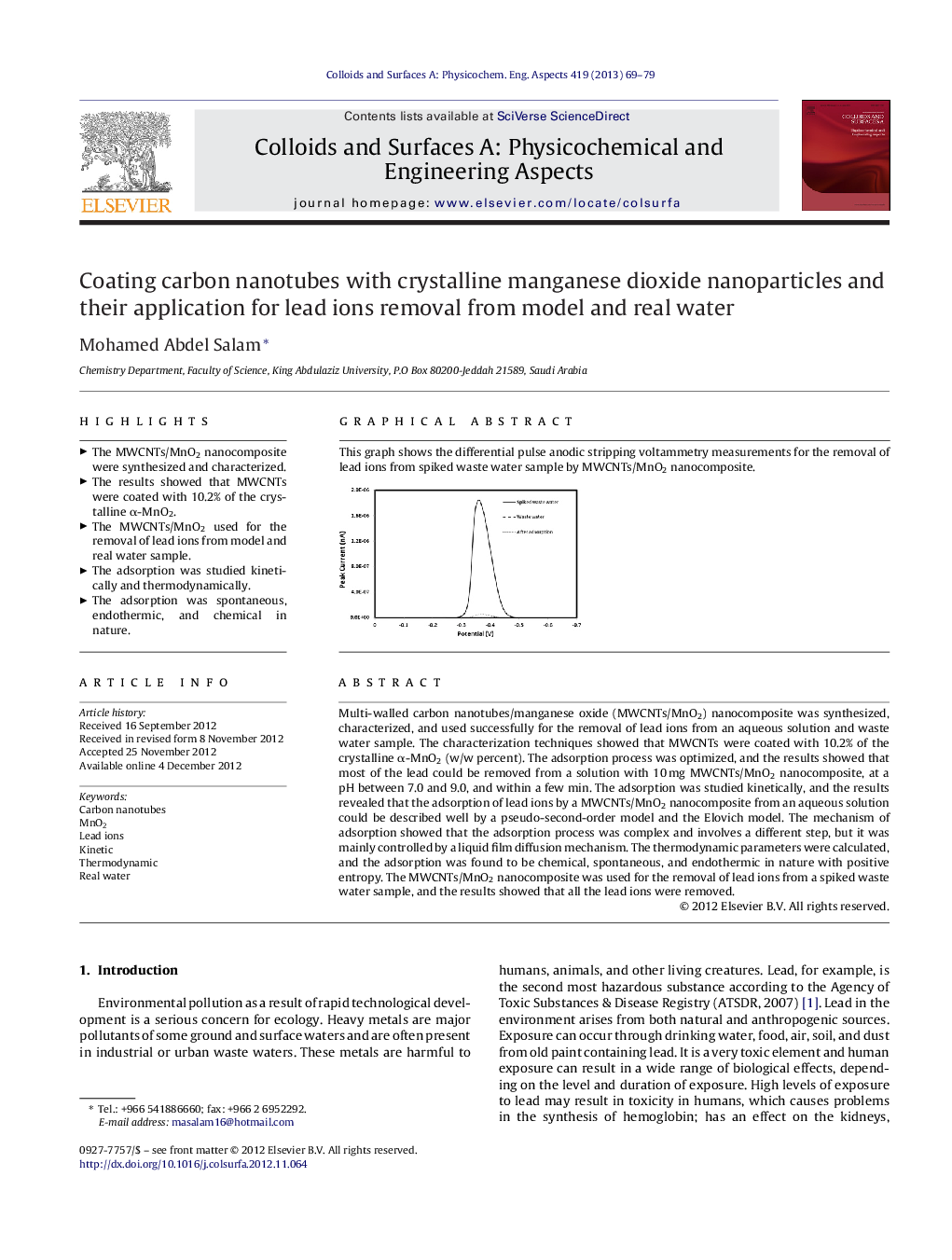
This graph shows the differential pulse anodic stripping voltammetry measurements for the removal of lead ions from spiked waste water sample by MWCNTs/MnO2 nanocomposite.Figure optionsDownload as PowerPoint slideHighlights
► The MWCNTs/MnO2 nanocomposite were synthesized and characterized.
► The results showed that MWCNTs were coated with 10.2% of the crystalline α-MnO2.
► The MWCNTs/MnO2 used for the removal of lead ions from model and real water sample.
► The adsorption was studied kinetically and thermodynamically.
► The adsorption was spontaneous, endothermic, and chemical in nature.
Journal: Colloids and Surfaces A: Physicochemical and Engineering Aspects - Volume 419, 20 February 2013, Pages 69–79