| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 593820 | 1453956 | 2013 | 10 صفحه PDF | دانلود رایگان |
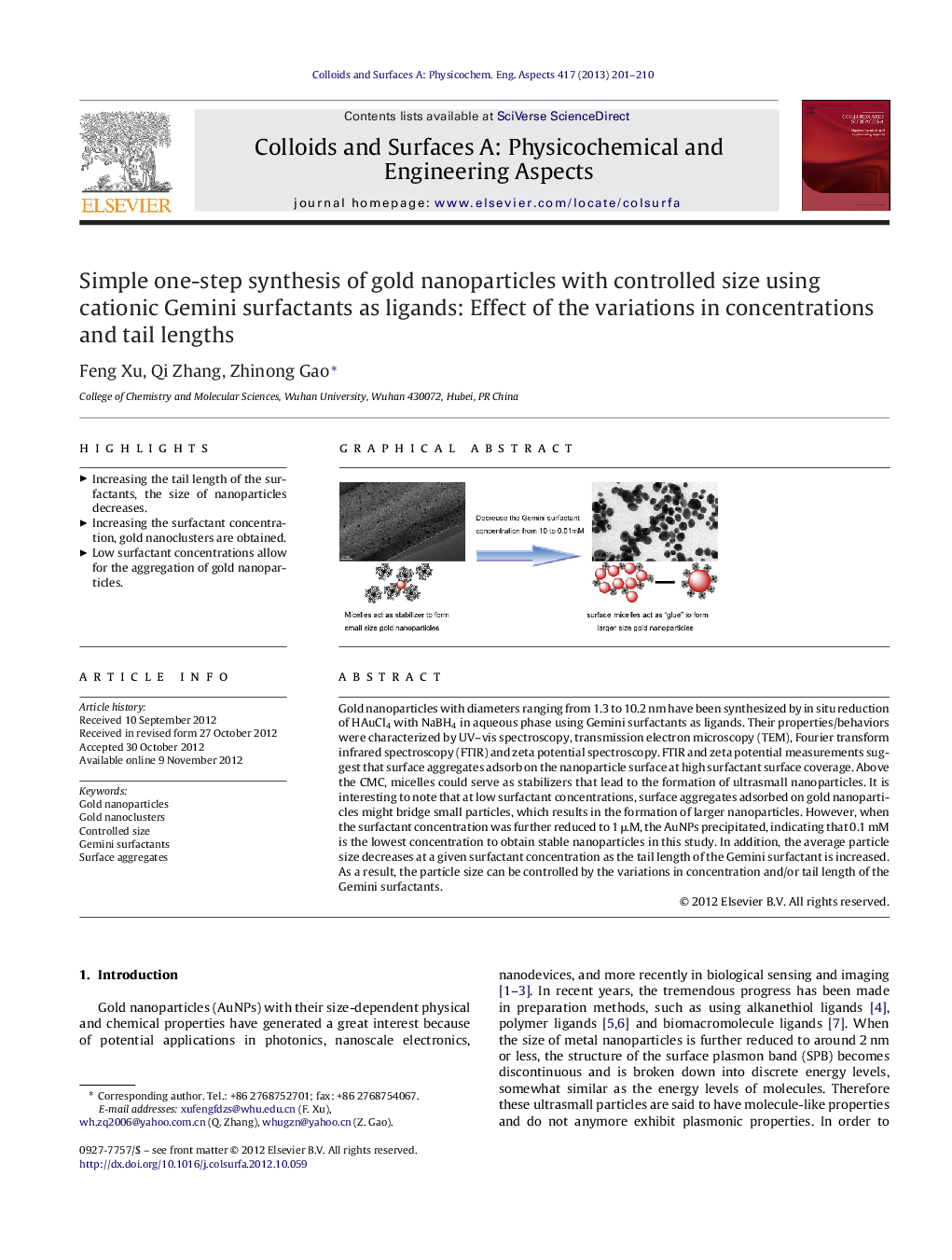
Gold nanoparticles with diameters ranging from 1.3 to 10.2 nm have been synthesized by in situ reduction of HAuCl4 with NaBH4 in aqueous phase using Gemini surfactants as ligands. Their properties/behaviors were characterized by UV–vis spectroscopy, transmission electron microscopy (TEM), Fourier transform infrared spectroscopy (FTIR) and zeta potential spectroscopy. FTIR and zeta potential measurements suggest that surface aggregates adsorb on the nanoparticle surface at high surfactant surface coverage. Above the CMC, micelles could serve as stabilizers that lead to the formation of ultrasmall nanoparticles. It is interesting to note that at low surfactant concentrations, surface aggregates adsorbed on gold nanoparticles might bridge small particles, which results in the formation of larger nanoparticles. However, when the surfactant concentration was further reduced to 1 μM, the AuNPs precipitated, indicating that 0.1 mM is the lowest concentration to obtain stable nanoparticles in this study. In addition, the average particle size decreases at a given surfactant concentration as the tail length of the Gemini surfactant is increased. As a result, the particle size can be controlled by the variations in concentration and/or tail length of the Gemini surfactants.
Figure optionsDownload as PowerPoint slideHighlights
► Increasing the tail length of the surfactants, the size of nanoparticles decreases.
► Increasing the surfactant concentration, gold nanoclusters are obtained.
► Low surfactant concentrations allow for the aggregation of gold nanoparticles.
Journal: Colloids and Surfaces A: Physicochemical and Engineering Aspects - Volume 417, 20 January 2013, Pages 201–210