| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 594040 | 1453963 | 2012 | 7 صفحه PDF | دانلود رایگان |
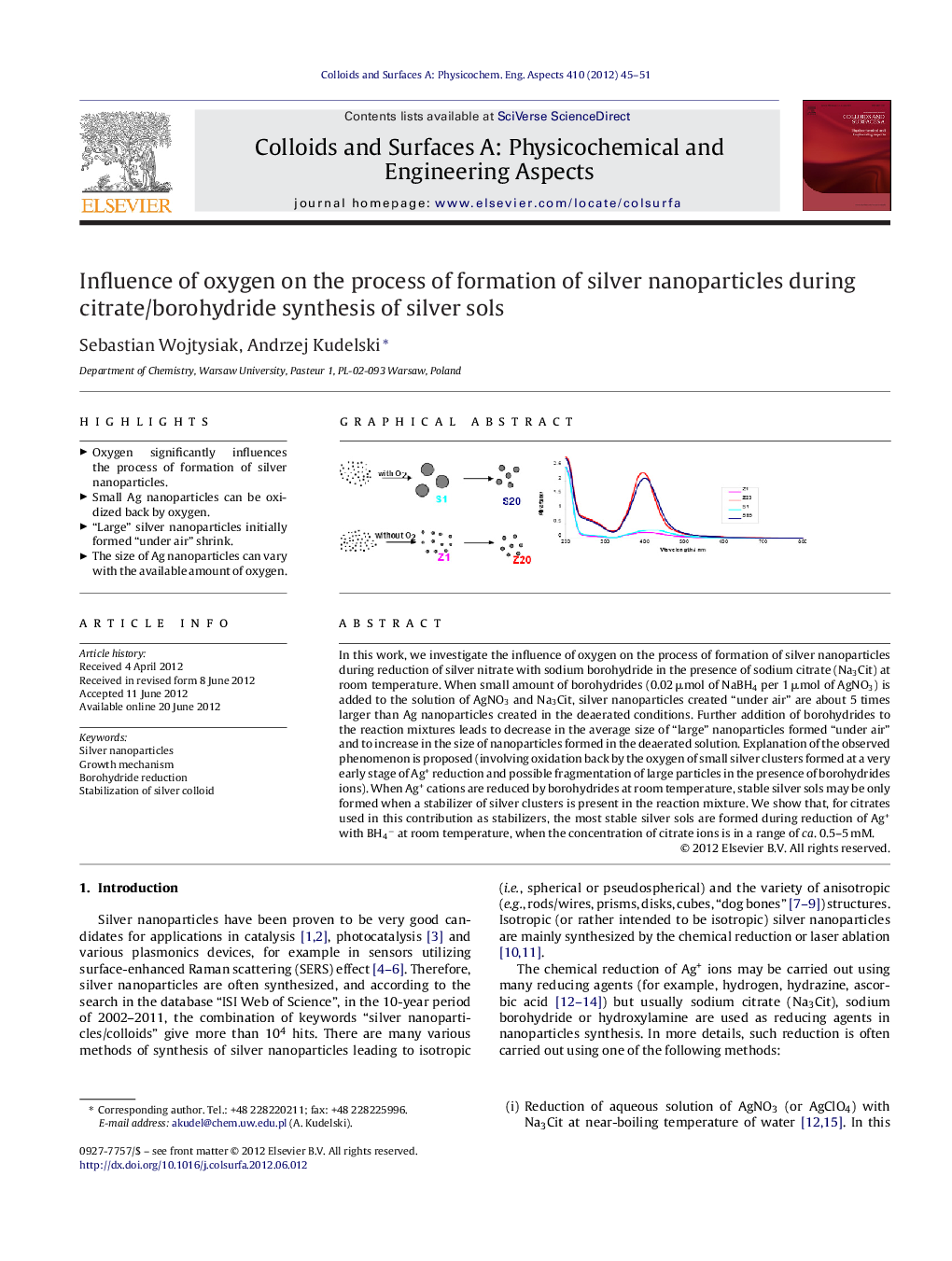
In this work, we investigate the influence of oxygen on the process of formation of silver nanoparticles during reduction of silver nitrate with sodium borohydride in the presence of sodium citrate (Na3Cit) at room temperature. When small amount of borohydrides (0.02 μmol of NaBH4 per 1 μmol of AgNO3) is added to the solution of AgNO3 and Na3Cit, silver nanoparticles created “under air” are about 5 times larger than Ag nanoparticles created in the deaerated conditions. Further addition of borohydrides to the reaction mixtures leads to decrease in the average size of “large” nanoparticles formed “under air” and to increase in the size of nanoparticles formed in the deaerated solution. Explanation of the observed phenomenon is proposed (involving oxidation back by the oxygen of small silver clusters formed at a very early stage of Ag+ reduction and possible fragmentation of large particles in the presence of borohydrides ions). When Ag+ cations are reduced by borohydrides at room temperature, stable silver sols may be only formed when a stabilizer of silver clusters is present in the reaction mixture. We show that, for citrates used in this contribution as stabilizers, the most stable silver sols are formed during reduction of Ag+ with BH4− at room temperature, when the concentration of citrate ions is in a range of ca. 0.5–5 mM.
Figure optionsDownload as PowerPoint slideHighlights
► Oxygen significantly influences the process of formation of silver nanoparticles.
► Small Ag nanoparticles can be oxidized back by oxygen.
► “Large” silver nanoparticles initially formed “under air” shrink.
► The size of Ag nanoparticles can vary with the available amount of oxygen.
Journal: Colloids and Surfaces A: Physicochemical and Engineering Aspects - Volume 410, 20 September 2012, Pages 45–51