| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 6309022 | 1618862 | 2014 | 7 صفحه PDF | دانلود رایگان |
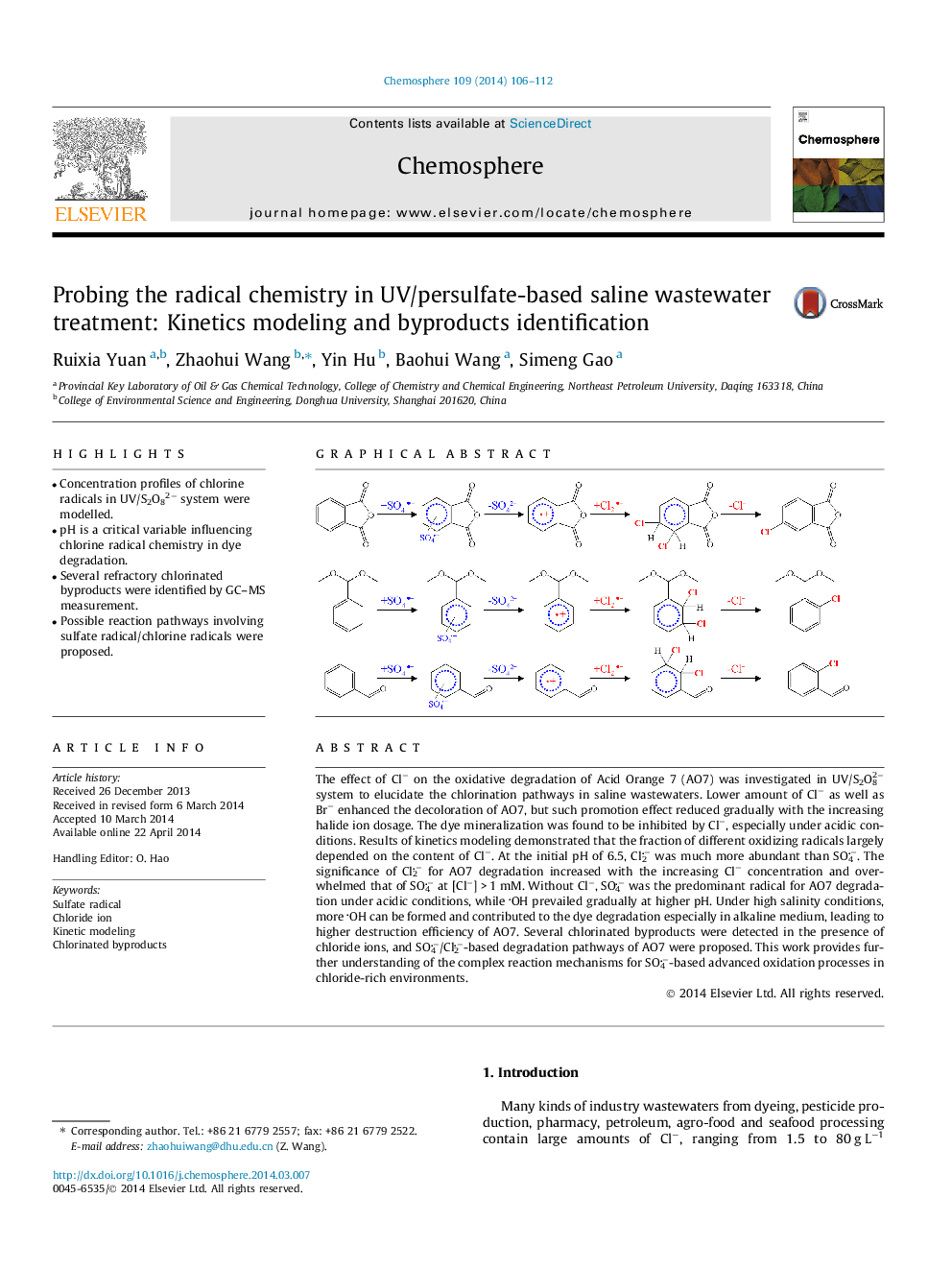
- Concentration profiles of chlorine radicals in UV/S2O82â system were modelled.
- pH is a critical variable influencing chlorine radical chemistry in dye degradation.
- Several refractory chlorinated byproducts were identified by GC-MS measurement.
- Possible reaction pathways involving sulfate radical/chlorine radicals were proposed.
The effect of Clâ on the oxidative degradation of Acid Orange 7 (AO7) was investigated in UV/S2O82â system to elucidate the chlorination pathways in saline wastewaters. Lower amount of Clâ as well as Brâ enhanced the decoloration of AO7, but such promotion effect reduced gradually with the increasing halide ion dosage. The dye mineralization was found to be inhibited by Clâ, especially under acidic conditions. Results of kinetics modeling demonstrated that the fraction of different oxidizing radicals largely depended on the content of Clâ. At the initial pH of 6.5, Cl2â was much more abundant than SO4â. The significance of Cl2â for AO7 degradation increased with the increasing Clâ concentration and overwhelmed that of SO4â at [Clâ]Â >Â 1Â mM. Without Clâ, SO4â was the predominant radical for AO7 degradation under acidic conditions, while OH prevailed gradually at higher pH. Under high salinity conditions, more OH can be formed and contributed to the dye degradation especially in alkaline medium, leading to higher destruction efficiency of AO7. Several chlorinated byproducts were detected in the presence of chloride ions, and SO4â/Cl2â-based degradation pathways of AO7 were proposed. This work provides further understanding of the complex reaction mechanisms for SO4â-based advanced oxidation processes in chloride-rich environments.
Journal: Chemosphere - Volume 109, August 2014, Pages 106-112