| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 6321868 | 1619726 | 2016 | 9 صفحه PDF | دانلود رایگان |
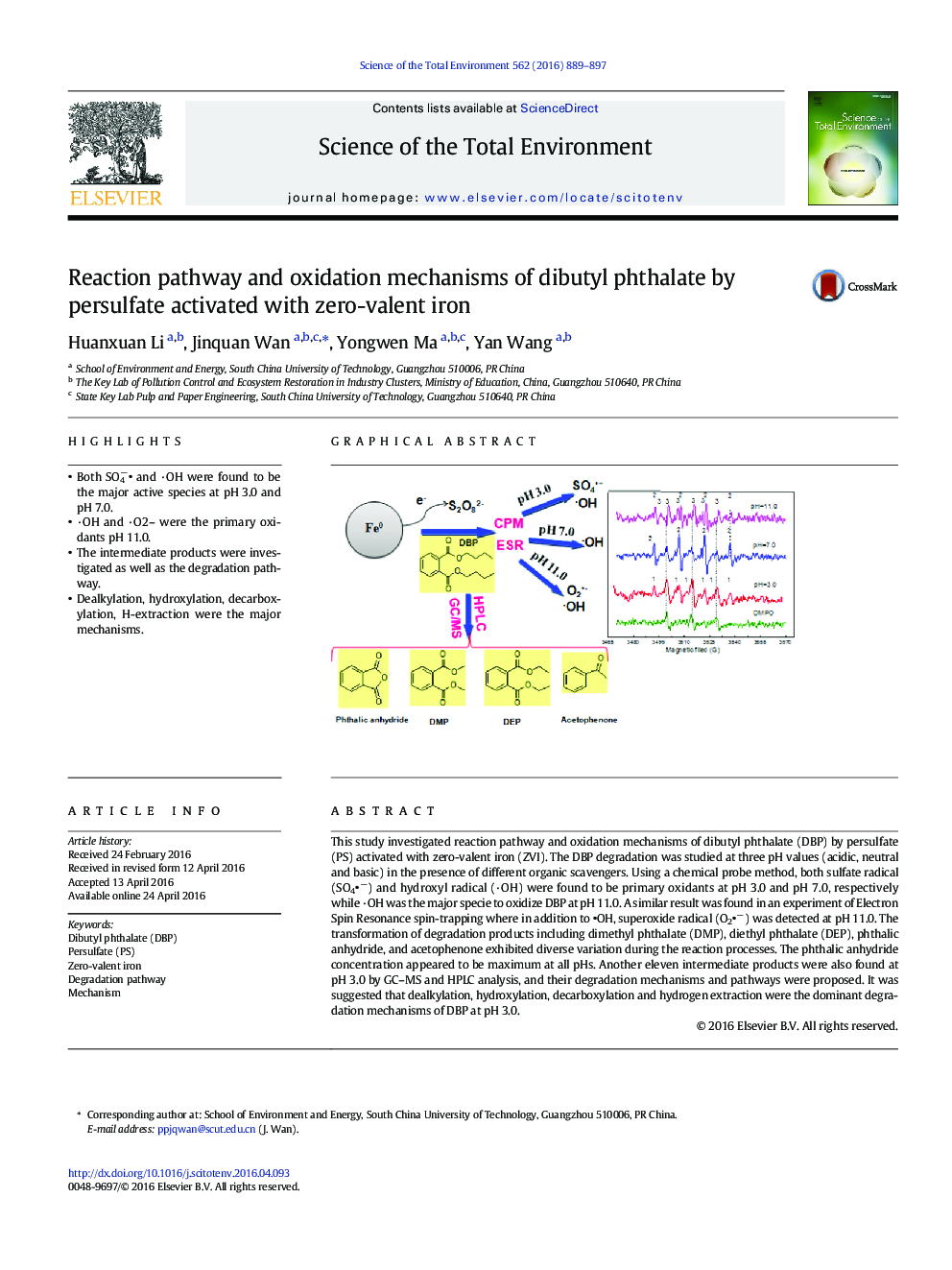
- Both SO4â and ·OH were found to be the major active species at pH 3.0 and pH 7.0.
- ·OH and ·O2- were the primary oxidants pH 11.0.
- The intermediate products were investigated as well as the degradation pathway.
- Dealkylation, hydroxylation, decarboxylation, H-extraction were the major mechanisms.
This study investigated reaction pathway and oxidation mechanisms of dibutyl phthalate (DBP) by persulfate (PS) activated with zero-valent iron (ZVI). The DBP degradation was studied at three pH values (acidic, neutral and basic) in the presence of different organic scavengers. Using a chemical probe method, both sulfate radical (SO4â) and hydroxyl radical (·OH) were found to be primary oxidants at pH 3.0 and pH 7.0, respectively while ·OH was the major specie to oxidize DBP at pH 11.0. A similar result was found in an experiment of Electron Spin Resonance spin-trapping where in addition to OH, superoxide radical (O2â) was detected at pH 11.0. The transformation of degradation products including dimethyl phthalate (DMP), diethyl phthalate (DEP), phthalic anhydride, and acetophenone exhibited diverse variation during the reaction processes. The phthalic anhydride concentration appeared to be maximum at all pHs. Another eleven intermediate products were also found at pH 3.0 by GC-MS and HPLC analysis, and their degradation mechanisms and pathways were proposed. It was suggested that dealkylation, hydroxylation, decarboxylation and hydrogen extraction were the dominant degradation mechanisms of DBP at pH 3.0.
115
Journal: Science of The Total Environment - Volume 562, 15 August 2016, Pages 889-897