| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 6465088 | 1422951 | 2017 | 9 صفحه PDF | دانلود رایگان |
- Lithium ferrites were positively tested as CO oxidants and subsequent CO2 captors.
- The bifunctional process was confirmed by the carbonation process.
- Li5FeO4 performed both processes in presence and absence of O2.
- LiFeO2 presents good properties for the CO oxidation, but not for the CO2 capture.
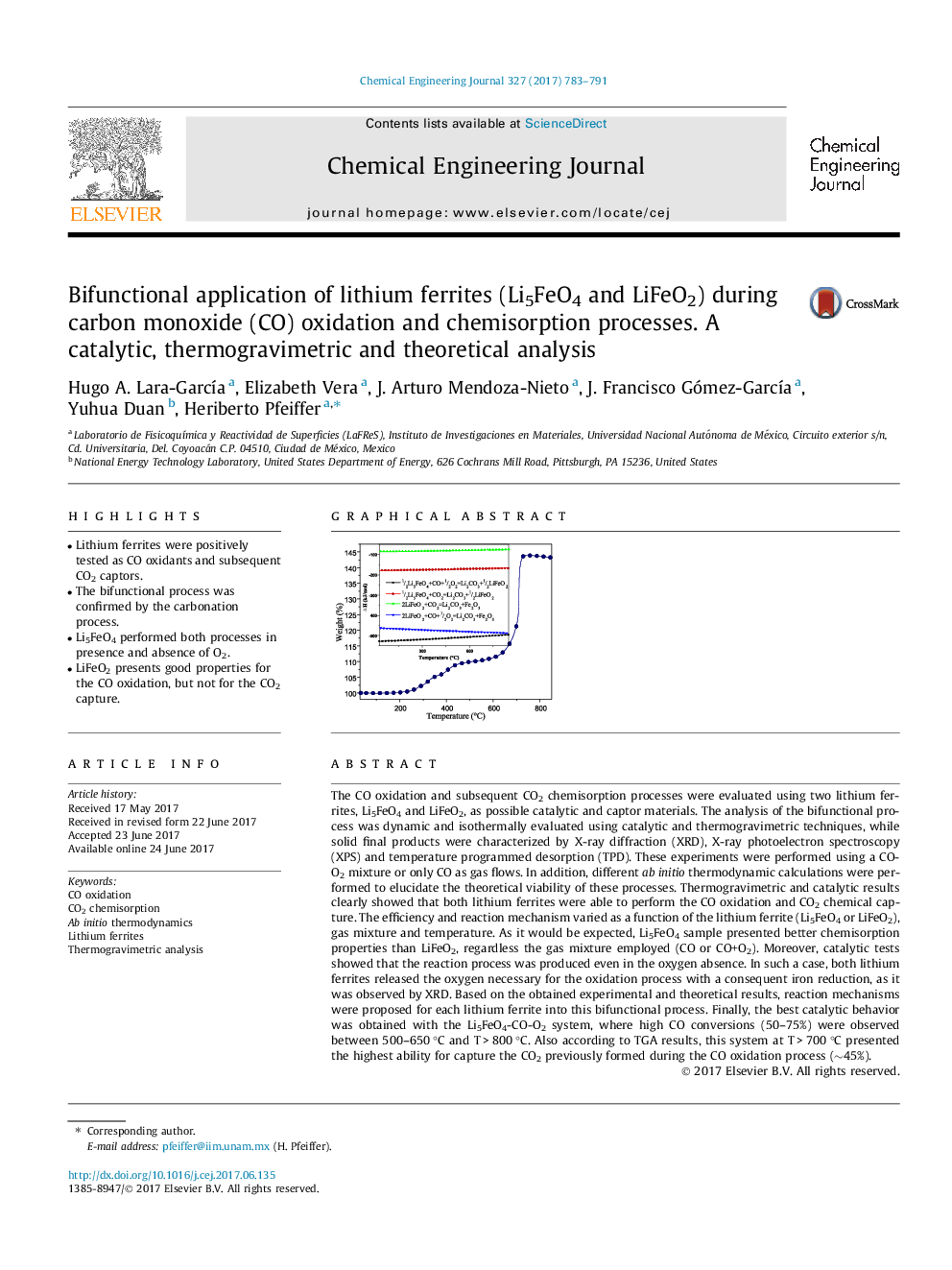
The CO oxidation and subsequent CO2 chemisorption processes were evaluated using two lithium ferrites, Li5FeO4 and LiFeO2, as possible catalytic and captor materials. The analysis of the bifunctional process was dynamic and isothermally evaluated using catalytic and thermogravimetric techniques, while solid final products were characterized by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS) and temperature programmed desorption (TPD). These experiments were performed using a CO-O2 mixture or only CO as gas flows. In addition, different ab initio thermodynamic calculations were performed to elucidate the theoretical viability of these processes. Thermogravimetric and catalytic results clearly showed that both lithium ferrites were able to perform the CO oxidation and CO2 chemical capture. The efficiency and reaction mechanism varied as a function of the lithium ferrite (Li5FeO4 or LiFeO2), gas mixture and temperature. As it would be expected, Li5FeO4 sample presented better chemisorption properties than LiFeO2, regardless the gas mixture employed (CO or CO+O2). Moreover, catalytic tests showed that the reaction process was produced even in the oxygen absence. In such a case, both lithium ferrites released the oxygen necessary for the oxidation process with a consequent iron reduction, as it was observed by XRD. Based on the obtained experimental and theoretical results, reaction mechanisms were proposed for each lithium ferrite into this bifunctional process. Finally, the best catalytic behavior was obtained with the Li5FeO4-CO-O2 system, where high CO conversions (50-75%) were observed between 500-650 °C and T > 800 °C. Also according to TGA results, this system at T > 700 °C presented the highest ability for capture the CO2 previously formed during the CO oxidation process (â¼45%).
119
Journal: Chemical Engineering Journal - Volume 327, 1 November 2017, Pages 783-791