| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 6465313 | 1422950 | 2017 | 13 صفحه PDF | دانلود رایگان |
- MoS2-NSs were synthesized by using eco-friendly and facile hydrothermal method.
- Covalent immobilization of β-amylase onto functionalized MoS2-NSs was investigated.
- Box-Behnken design optimized parameters, resulting into 92% immobilization efficiency.
- XRD, RAMAN, SEM, TEM, FT-IR, AFM and confocal microscopy confirmed immobilization.
- Improvement of steady state kinetics of β-amylase was observed due to immobilization.
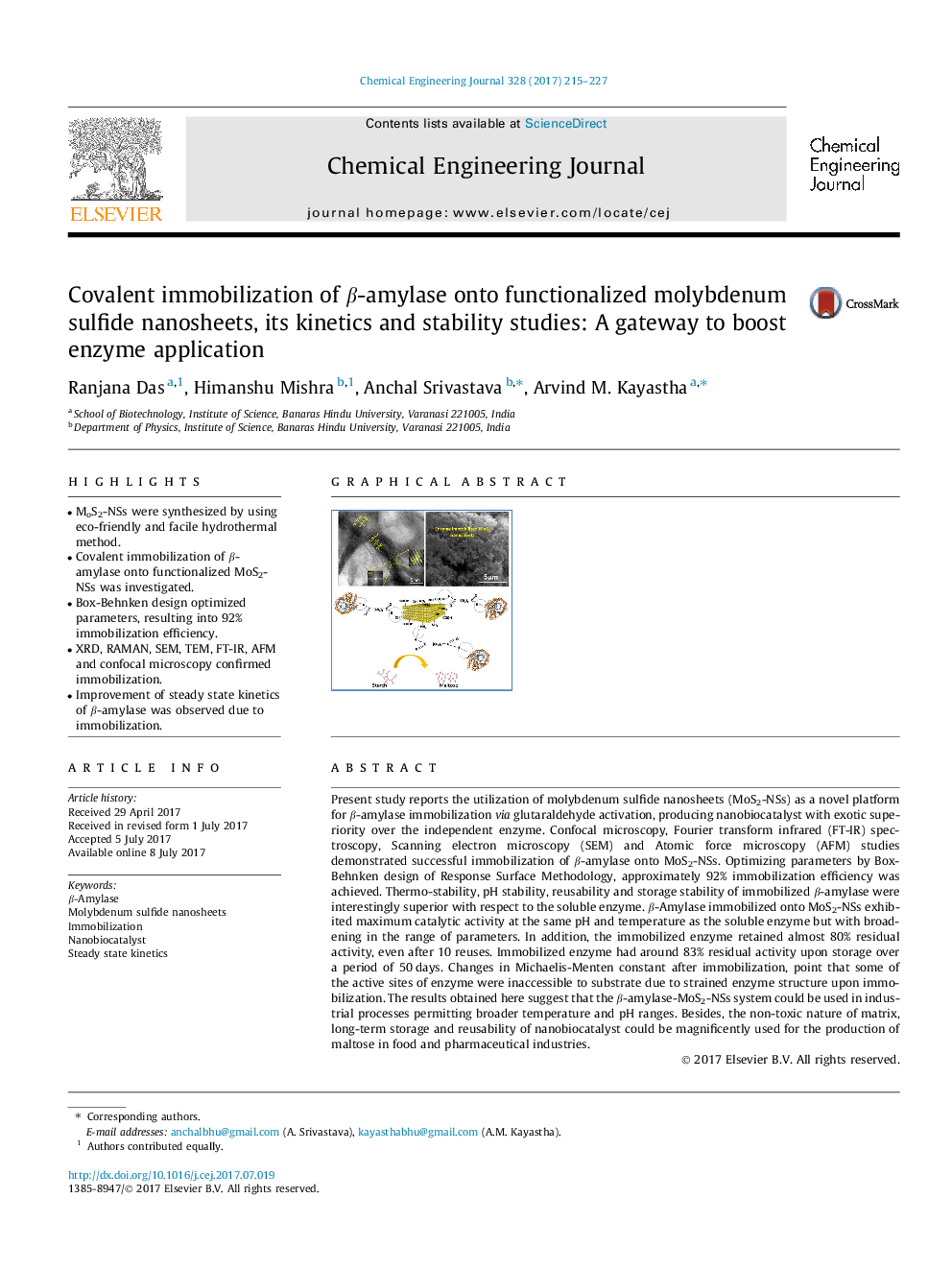
Present study reports the utilization of molybdenum sulfide nanosheets (MoS2-NSs) as a novel platform for β-amylase immobilization via glutaraldehyde activation, producing nanobiocatalyst with exotic superiority over the independent enzyme. Confocal microscopy, Fourier transform infrared (FT-IR) spectroscopy, Scanning electron microscopy (SEM) and Atomic force microscopy (AFM) studies demonstrated successful immobilization of β-amylase onto MoS2-NSs. Optimizing parameters by Box-Behnken design of Response Surface Methodology, approximately 92% immobilization efficiency was achieved. Thermo-stability, pH stability, reusability and storage stability of immobilized β-amylase were interestingly superior with respect to the soluble enzyme. β-Amylase immobilized onto MoS2-NSs exhibited maximum catalytic activity at the same pH and temperature as the soluble enzyme but with broadening in the range of parameters. In addition, the immobilized enzyme retained almost 80% residual activity, even after 10 reuses. Immobilized enzyme had around 83% residual activity upon storage over a period of 50 days. Changes in Michaelis-Menten constant after immobilization, point that some of the active sites of enzyme were inaccessible to substrate due to strained enzyme structure upon immobilization. The results obtained here suggest that the β-amylase-MoS2-NSs system could be used in industrial processes permitting broader temperature and pH ranges. Besides, the non-toxic nature of matrix, long-term storage and reusability of nanobiocatalyst could be magnificently used for the production of maltose in food and pharmaceutical industries.
86
Journal: Chemical Engineering Journal - Volume 328, 15 November 2017, Pages 215-227