| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 6465951 | 1422958 | 2017 | 12 صفحه PDF | دانلود رایگان |
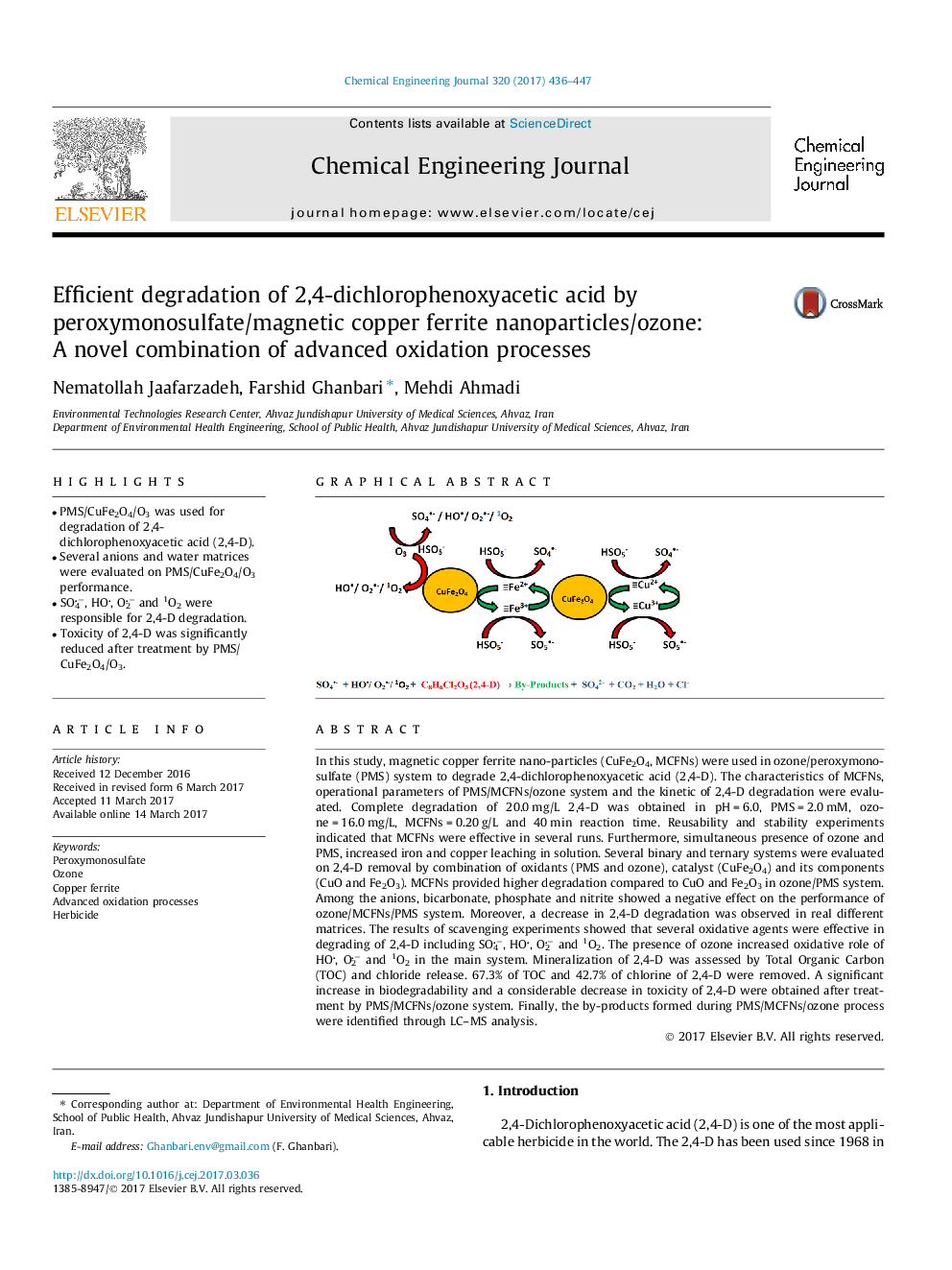
- PMS/CuFe2O4/O3 was used for degradation of 2,4-dichlorophenoxyacetic acid (2,4-D).
- Several anions and water matrices were evaluated on PMS/CuFe2O4/O3 performance.
- SO4â, HO, O2â and 1O2 were responsible for 2,4-D degradation.
- Toxicity of 2,4-D was significantly reduced after treatment by PMS/CuFe2O4/O3.
In this study, magnetic copper ferrite nano-particles (CuFe2O4, MCFNs) were used in ozone/peroxymonosulfate (PMS) system to degrade 2,4-dichlorophenoxyacetic acid (2,4-D). The characteristics of MCFNs, operational parameters of PMS/MCFNs/ozone system and the kinetic of 2,4-D degradation were evaluated. Complete degradation of 20.0 mg/L 2,4-D was obtained in pH = 6.0, PMS = 2.0 mM, ozone = 16.0 mg/L, MCFNs = 0.20 g/L and 40 min reaction time. Reusability and stability experiments indicated that MCFNs were effective in several runs. Furthermore, simultaneous presence of ozone and PMS, increased iron and copper leaching in solution. Several binary and ternary systems were evaluated on 2,4-D removal by combination of oxidants (PMS and ozone), catalyst (CuFe2O4) and its components (CuO and Fe2O3). MCFNs provided higher degradation compared to CuO and Fe2O3 in ozone/PMS system. Among the anions, bicarbonate, phosphate and nitrite showed a negative effect on the performance of ozone/MCFNs/PMS system. Moreover, a decrease in 2,4-D degradation was observed in real different matrices. The results of scavenging experiments showed that several oxidative agents were effective in degrading of 2,4-D including SO4â, HO, O2â and 1O2. The presence of ozone increased oxidative role of HO, O2â and 1O2 in the main system. Mineralization of 2,4-D was assessed by Total Organic Carbon (TOC) and chloride release. 67.3% of TOC and 42.7% of chlorine of 2,4-D were removed. A significant increase in biodegradability and a considerable decrease in toxicity of 2,4-D were obtained after treatment by PMS/MCFNs/ozone system. Finally, the by-products formed during PMS/MCFNs/ozone process were identified through LC-MS analysis.
81
Journal: Chemical Engineering Journal - Volume 320, 15 July 2017, Pages 436-447