| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 6466655 | 1422965 | 2017 | 10 صفحه PDF | دانلود رایگان |
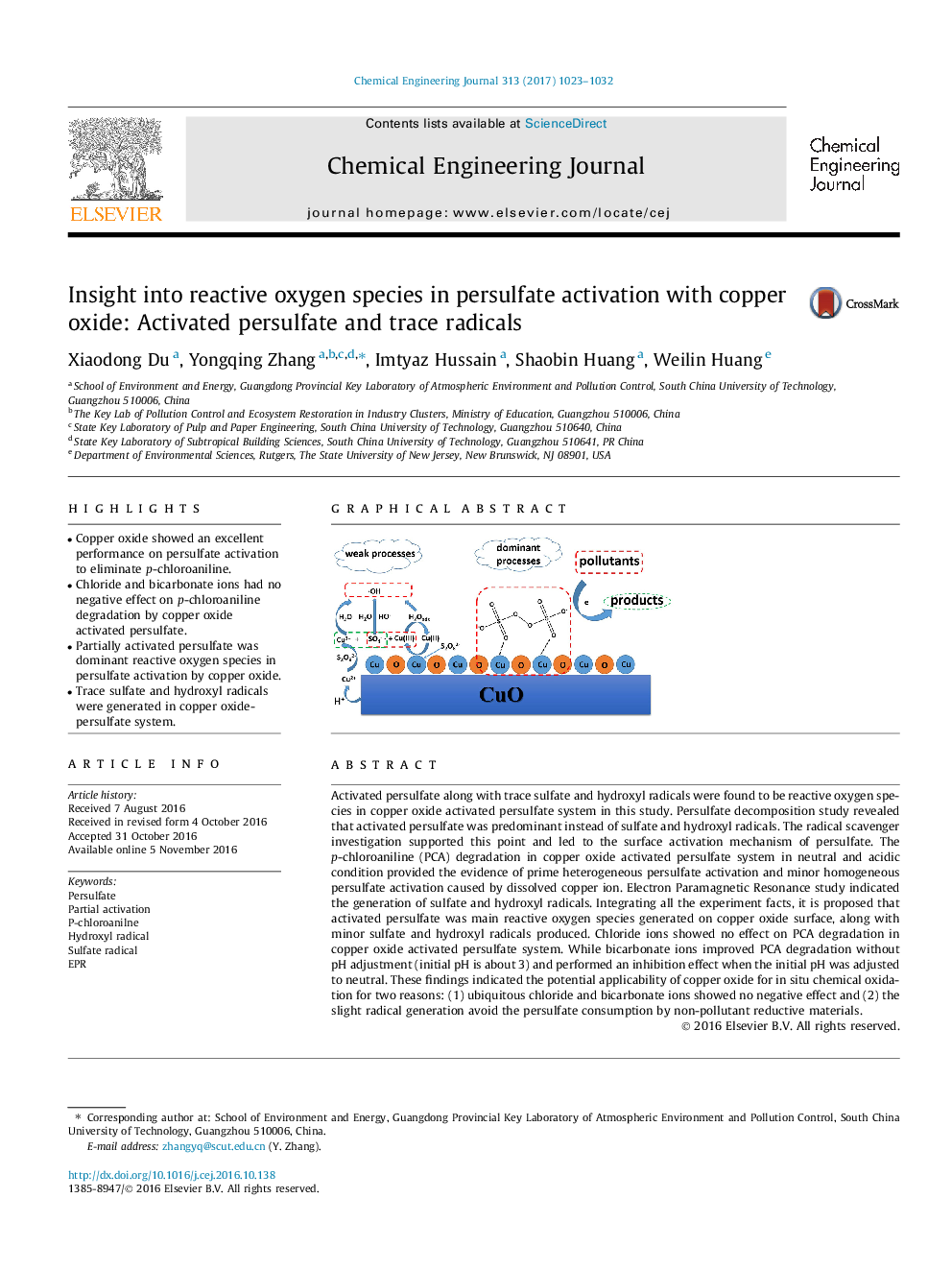
- Copper oxide showed an excellent performance on persulfate activation to eliminate p-chloroaniline.
- Chloride and bicarbonate ions had no negative effect on p-chloroaniline degradation by copper oxide activated persulfate.
- Partially activated persulfate was dominant reactive oxygen species in persulfate activation by copper oxide.
- Trace sulfate and hydroxyl radicals were generated in copper oxide-persulfate system.
Activated persulfate along with trace sulfate and hydroxyl radicals were found to be reactive oxygen species in copper oxide activated persulfate system in this study. Persulfate decomposition study revealed that activated persulfate was predominant instead of sulfate and hydroxyl radicals. The radical scavenger investigation supported this point and led to the surface activation mechanism of persulfate. The p-chloroaniline (PCA) degradation in copper oxide activated persulfate system in neutral and acidic condition provided the evidence of prime heterogeneous persulfate activation and minor homogeneous persulfate activation caused by dissolved copper ion. Electron Paramagnetic Resonance study indicated the generation of sulfate and hydroxyl radicals. Integrating all the experiment facts, it is proposed that activated persulfate was main reactive oxygen species generated on copper oxide surface, along with minor sulfate and hydroxyl radicals produced. Chloride ions showed no effect on PCA degradation in copper oxide activated persulfate system. While bicarbonate ions improved PCA degradation without pH adjustment (initial pH is about 3) and performed an inhibition effect when the initial pH was adjusted to neutral. These findings indicated the potential applicability of copper oxide for in situ chemical oxidation for two reasons: (1) ubiquitous chloride and bicarbonate ions showed no negative effect and (2) the slight radical generation avoid the persulfate consumption by non-pollutant reductive materials.
67
Journal: Chemical Engineering Journal - Volume 313, 1 April 2017, Pages 1023-1032