| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 10965227 | 1102739 | 2014 | 4 صفحه PDF | دانلود رایگان |
عنوان انگلیسی مقاله ISI
Implementation of QbD for the development of a vaccine candidate
دانلود مقاله + سفارش ترجمه
دانلود مقاله ISI انگلیسی
رایگان برای ایرانیان
موضوعات مرتبط
علوم زیستی و بیوفناوری
ایمنی شناسی و میکروب شناسی
ایمونولوژی
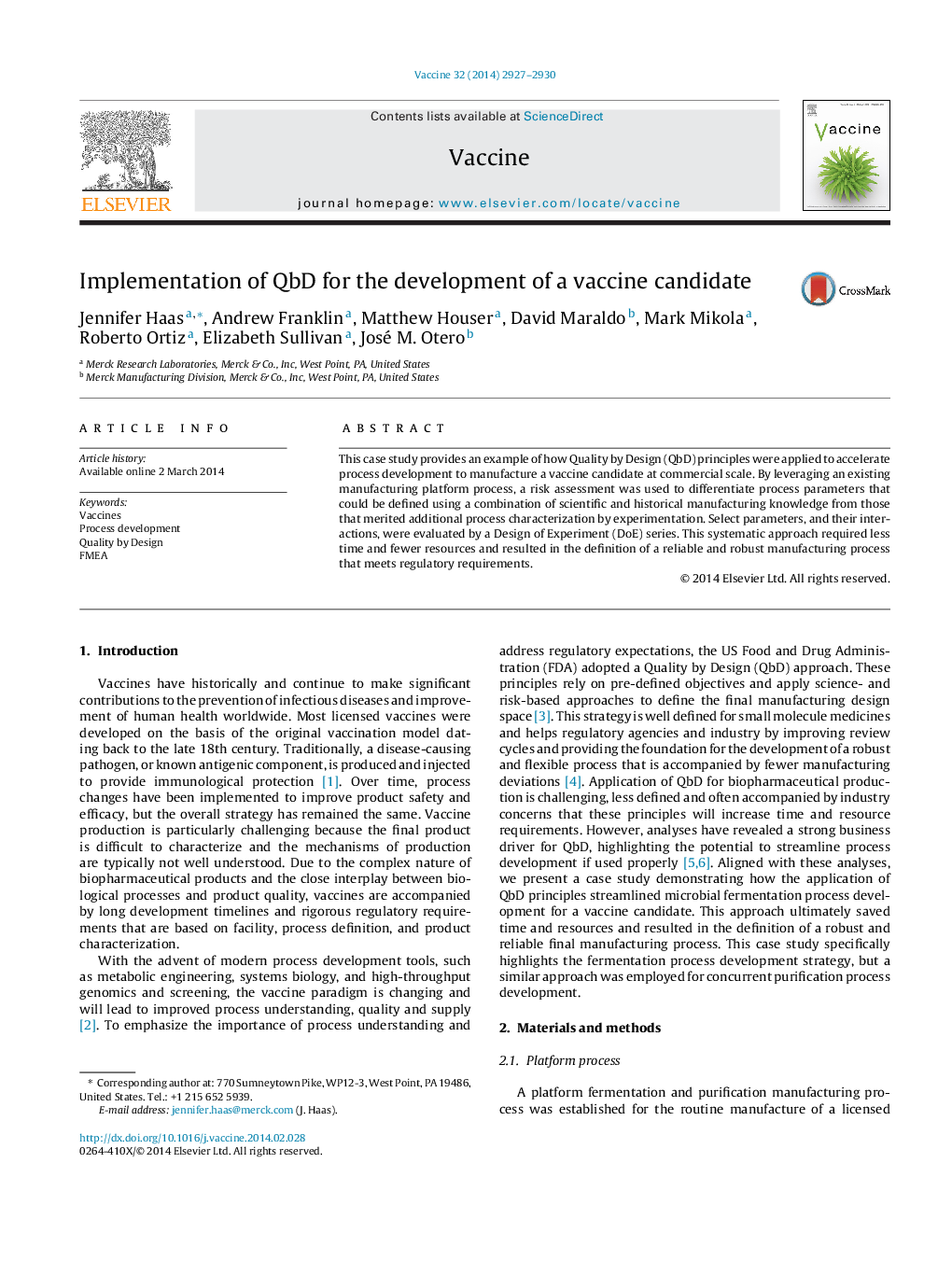
پیش نمایش صفحه اول مقاله
چکیده انگلیسی
This case study provides an example of how Quality by Design (QbD) principles were applied to accelerate process development to manufacture a vaccine candidate at commercial scale. By leveraging an existing manufacturing platform process, a risk assessment was used to differentiate process parameters that could be defined using a combination of scientific and historical manufacturing knowledge from those that merited additional process characterization by experimentation. Select parameters, and their interactions, were evaluated by a Design of Experiment (DoE) series. This systematic approach required less time and fewer resources and resulted in the definition of a reliable and robust manufacturing process that meets regulatory requirements.
ناشر
Database: Elsevier - ScienceDirect (ساینس دایرکت)
Journal: Vaccine - Volume 32, Issue 24, 19 May 2014, Pages 2927-2930
Journal: Vaccine - Volume 32, Issue 24, 19 May 2014, Pages 2927-2930
نویسندگان
Jennifer Haas, Andrew Franklin, Matthew Houser, David Maraldo, Mark Mikola, Roberto Ortiz, Elizabeth Sullivan, José M. Otero,