| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1163089 | 1490923 | 2016 | 8 صفحه PDF | دانلود رایگان |
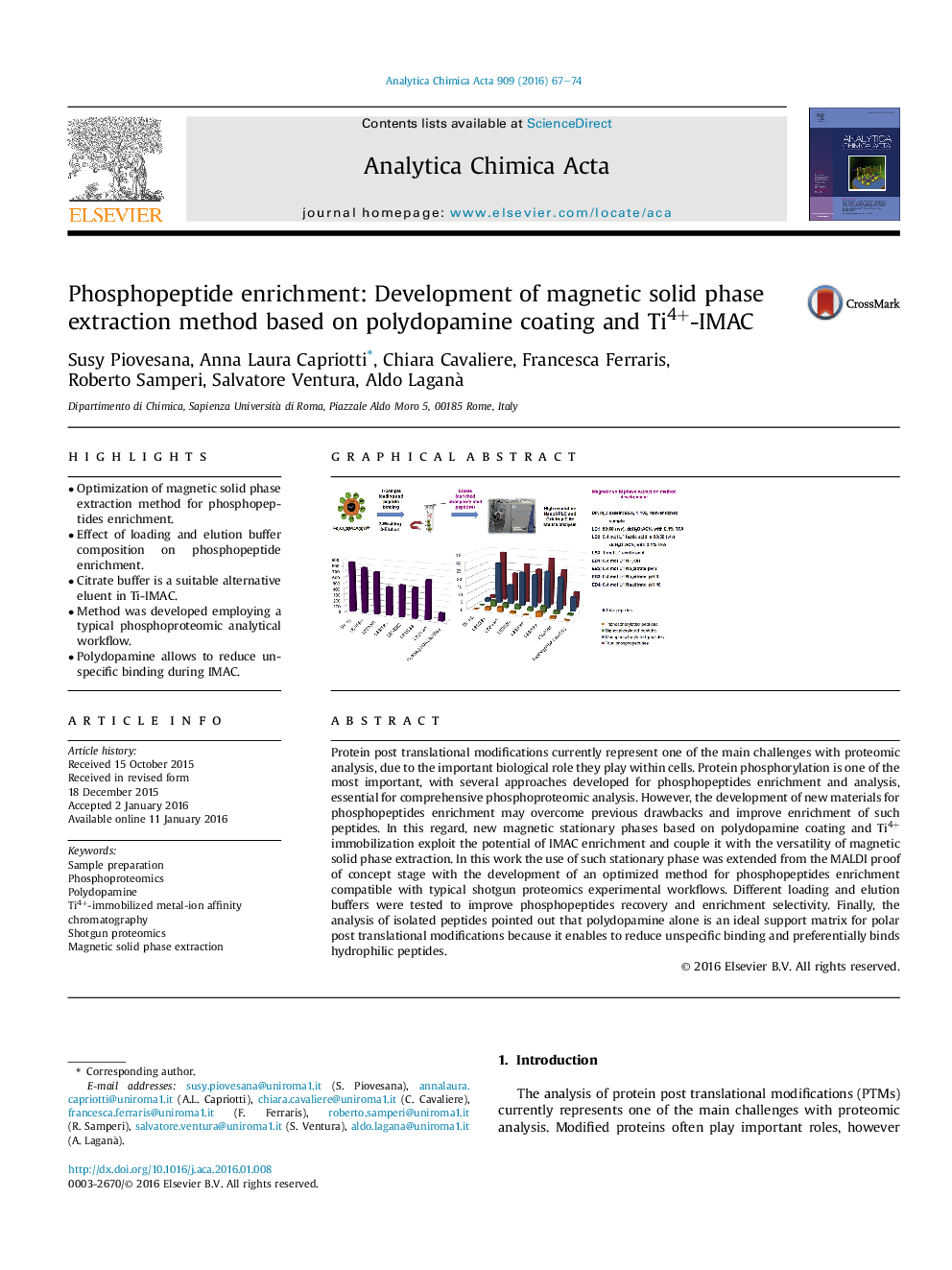
• Optimization of magnetic solid phase extraction method for phosphopeptides enrichment.
• Effect of loading and elution buffer composition on phosphopeptide enrichment.
• Citrate buffer is a suitable alternative eluent in Ti-IMAC.
• Method was developed employing a typical phosphoproteomic analytical workflow.
• Polydopamine allows to reduce unspecific binding during IMAC.
Protein post translational modifications currently represent one of the main challenges with proteomic analysis, due to the important biological role they play within cells. Protein phosphorylation is one of the most important, with several approaches developed for phosphopeptides enrichment and analysis, essential for comprehensive phosphoproteomic analysis. However, the development of new materials for phosphopeptides enrichment may overcome previous drawbacks and improve enrichment of such peptides. In this regard, new magnetic stationary phases based on polydopamine coating and Ti4+ immobilization exploit the potential of IMAC enrichment and couple it with the versatility of magnetic solid phase extraction. In this work the use of such stationary phase was extended from the MALDI proof of concept stage with the development of an optimized method for phosphopeptides enrichment compatible with typical shotgun proteomics experimental workflows. Different loading and elution buffers were tested to improve phosphopeptides recovery and enrichment selectivity. Finally, the analysis of isolated peptides pointed out that polydopamine alone is an ideal support matrix for polar post translational modifications because it enables to reduce unspecific binding and preferentially binds hydrophilic peptides.
Figure optionsDownload as PowerPoint slide
Journal: Analytica Chimica Acta - Volume 909, 25 February 2016, Pages 67–74