| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1165142 | 1491081 | 2012 | 8 صفحه PDF | دانلود رایگان |
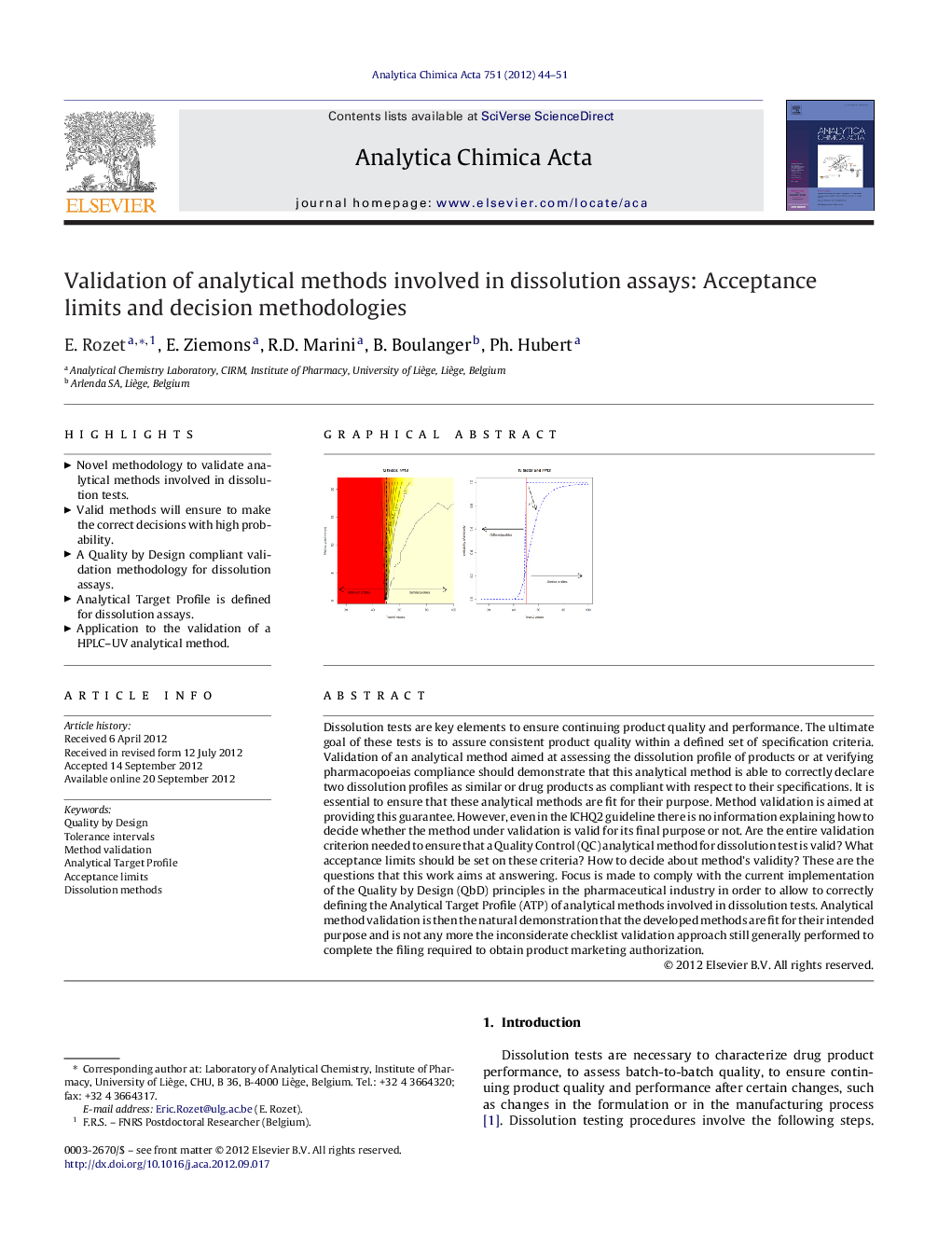
Dissolution tests are key elements to ensure continuing product quality and performance. The ultimate goal of these tests is to assure consistent product quality within a defined set of specification criteria. Validation of an analytical method aimed at assessing the dissolution profile of products or at verifying pharmacopoeias compliance should demonstrate that this analytical method is able to correctly declare two dissolution profiles as similar or drug products as compliant with respect to their specifications. It is essential to ensure that these analytical methods are fit for their purpose. Method validation is aimed at providing this guarantee. However, even in the ICHQ2 guideline there is no information explaining how to decide whether the method under validation is valid for its final purpose or not. Are the entire validation criterion needed to ensure that a Quality Control (QC) analytical method for dissolution test is valid? What acceptance limits should be set on these criteria? How to decide about method's validity? These are the questions that this work aims at answering. Focus is made to comply with the current implementation of the Quality by Design (QbD) principles in the pharmaceutical industry in order to allow to correctly defining the Analytical Target Profile (ATP) of analytical methods involved in dissolution tests. Analytical method validation is then the natural demonstration that the developed methods are fit for their intended purpose and is not any more the inconsiderate checklist validation approach still generally performed to complete the filing required to obtain product marketing authorization.
Figure optionsDownload as PowerPoint slideHighlights
► Novel methodology to validate analytical methods involved in dissolution tests.
► Valid methods will ensure to make the correct decisions with high probability.
► A Quality by Design compliant validation methodology for dissolution assays.
► Analytical Target Profile is defined for dissolution assays.
► Application to the validation of a HPLC–UV analytical method.
Journal: Analytica Chimica Acta - Volume 751, 2 November 2012, Pages 44–51