| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1165847 | 1491085 | 2012 | 7 صفحه PDF | دانلود رایگان |
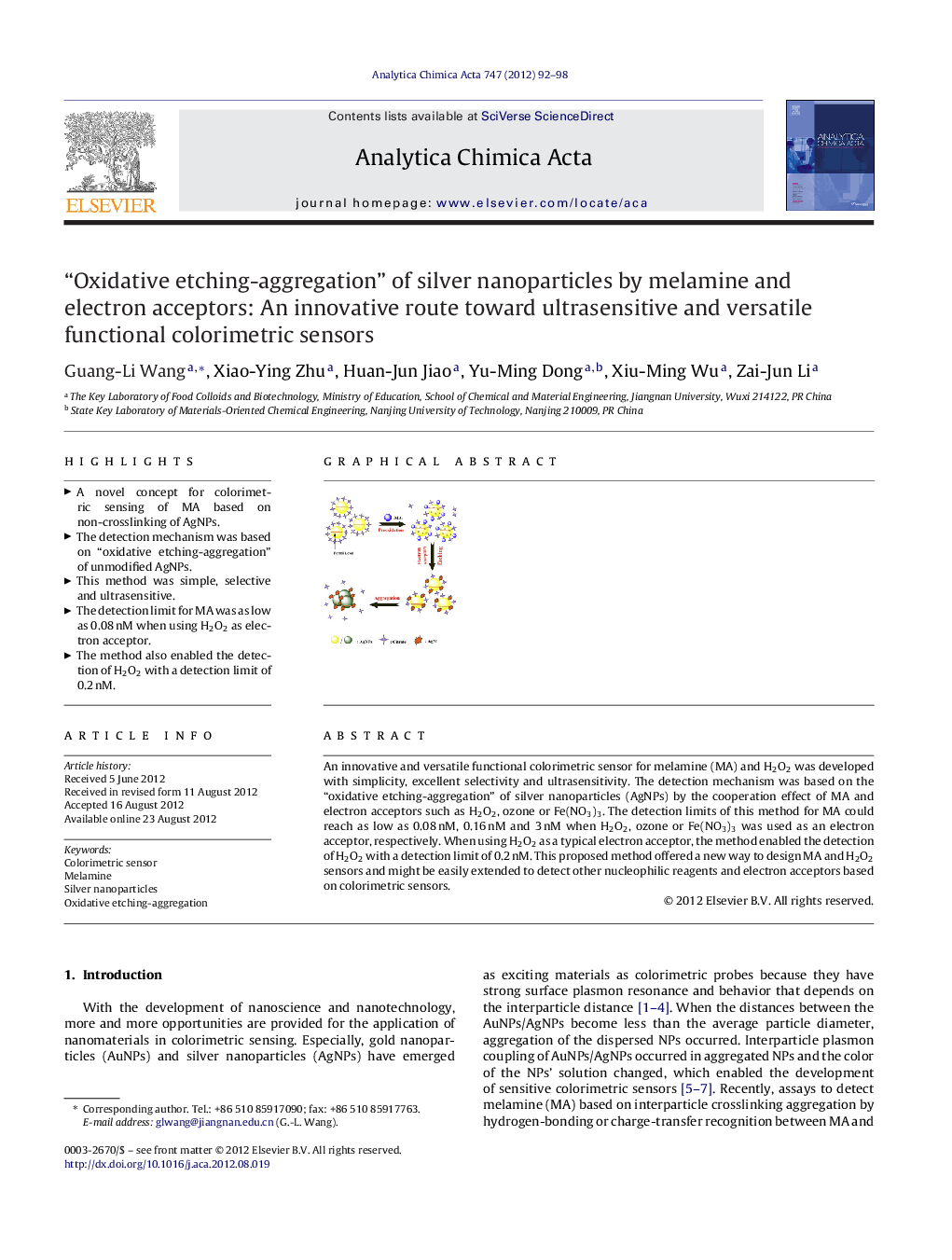
An innovative and versatile functional colorimetric sensor for melamine (MA) and H2O2 was developed with simplicity, excellent selectivity and ultrasensitivity. The detection mechanism was based on the “oxidative etching-aggregation” of silver nanoparticles (AgNPs) by the cooperation effect of MA and electron acceptors such as H2O2, ozone or Fe(NO3)3. The detection limits of this method for MA could reach as low as 0.08 nM, 0.16 nM and 3 nM when H2O2, ozone or Fe(NO3)3 was used as an electron acceptor, respectively. When using H2O2 as a typical electron acceptor, the method enabled the detection of H2O2 with a detection limit of 0.2 nM. This proposed method offered a new way to design MA and H2O2 sensors and might be easily extended to detect other nucleophilic reagents and electron acceptors based on colorimetric sensors.
Figure optionsDownload as PowerPoint slideHighlights
► A novel concept for colorimetric sensing of MA based on non-crosslinking of AgNPs.
► The detection mechanism was based on “oxidative etching-aggregation” of unmodified AgNPs.
► This method was simple, selective and ultrasensitive.
► The detection limit for MA was as low as 0.08 nM when using H2O2 as electron acceptor.
► The method also enabled the detection of H2O2 with a detection limit of 0.2 nM.
Journal: Analytica Chimica Acta - Volume 747, 17 October 2012, Pages 92–98