| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1355224 | 980827 | 2011 | 14 صفحه PDF | دانلود رایگان |
The dependence of the solution structure of neamine on pH was determined by NMR and AMBER molecular dynamics methods at pD 3.3, pD 6.5, and pD 7.4 in D2O at 25 °C. Unlike neamine structures at pD 3.3 and 6.5, which essentially showed only one conformer, slowly exchanging primary, P-state, and secondary, S-state, neamine conformers populated on the NMR time scale at ∼80% and ∼20%, respectively, were detected at pD 7.4 with kinetic constants kon(P→S) = 1.9771 s−1 and koff(S→P) = 1.1319 s−1. A tertiary, T-state, neamine species populated at ∼3% was also detected by NMR at pD 7.4. The pKa values determined by NMR titration experiments are pKa1 6.44 ± 0.13 for N3 of ring-II, pKa2 7.23 ± 0.09 for N2′ of ring-I, pKa3 7.77 ± 0.19 for N1 of ring-II, and pKa4 8.08 ± 0.15 for N6′ of ring-I. Ring-I and ring-II of the P-state neamine and ring-I of the S and T-states of neamine possess the 4C1 chair conformation between pD 3.3 and pD = 7.4. In contrast, ring-II of the S and T-states of neamine most likely adopt the 6rH1 half-chair conformation. The P and S-states of neamine exhibit a negative syn-ψ glycosidic geometry. The exocyclic aminomethyl group of ring-I adopts the gt exocyclic rotamer conformation around physiological pHs while the gg exocyclic rotamer conformation predominates in acidic solutions near and below pH 4.5. Neamine exists in the P-state as a mixture of tetra-/tri-/di-protonated species between pD 4.5 and pD 7.4, while the S-state neamine exist only in a di-protonated species around physiological pDs. The existence of the S-state neamine may facilitate binding of neamine-like aminoglycosides by favorable entropy of binding to the A-site of 16S ribosomal RNA, suggesting that novel aminoglycoside compounds carrying a S-state neamine pharmacophore can be developed.
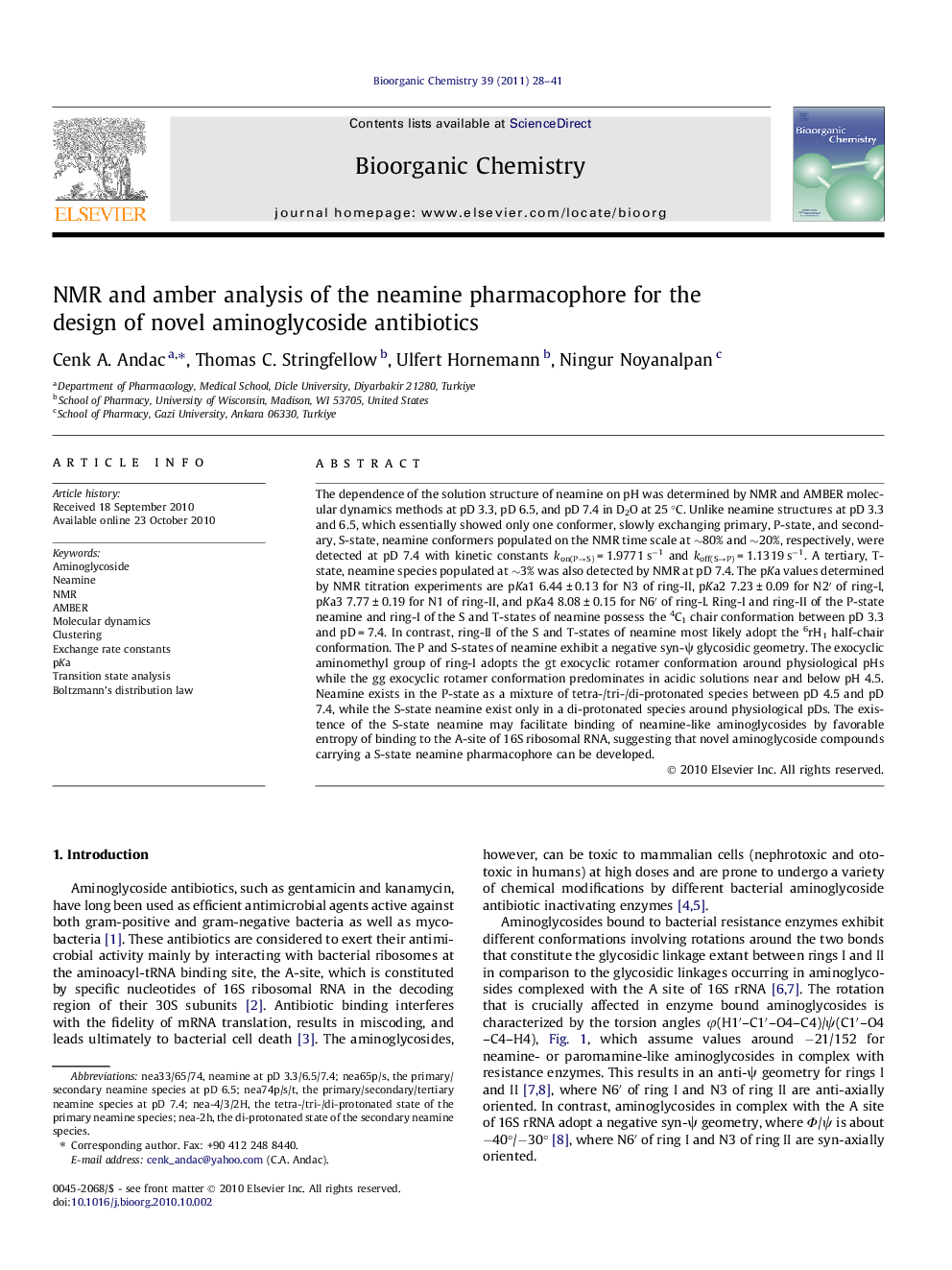
Slow conformational exchange between the primary (P) and secondary (S) states of the neamine pharmacophore. Hydrogens are not shown for simplicity..Figure optionsDownload as PowerPoint slide
Journal: Bioorganic Chemistry - Volume 39, Issue 1, February 2011, Pages 28–41