| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1364876 | 981548 | 2007 | 6 صفحه PDF | دانلود رایگان |
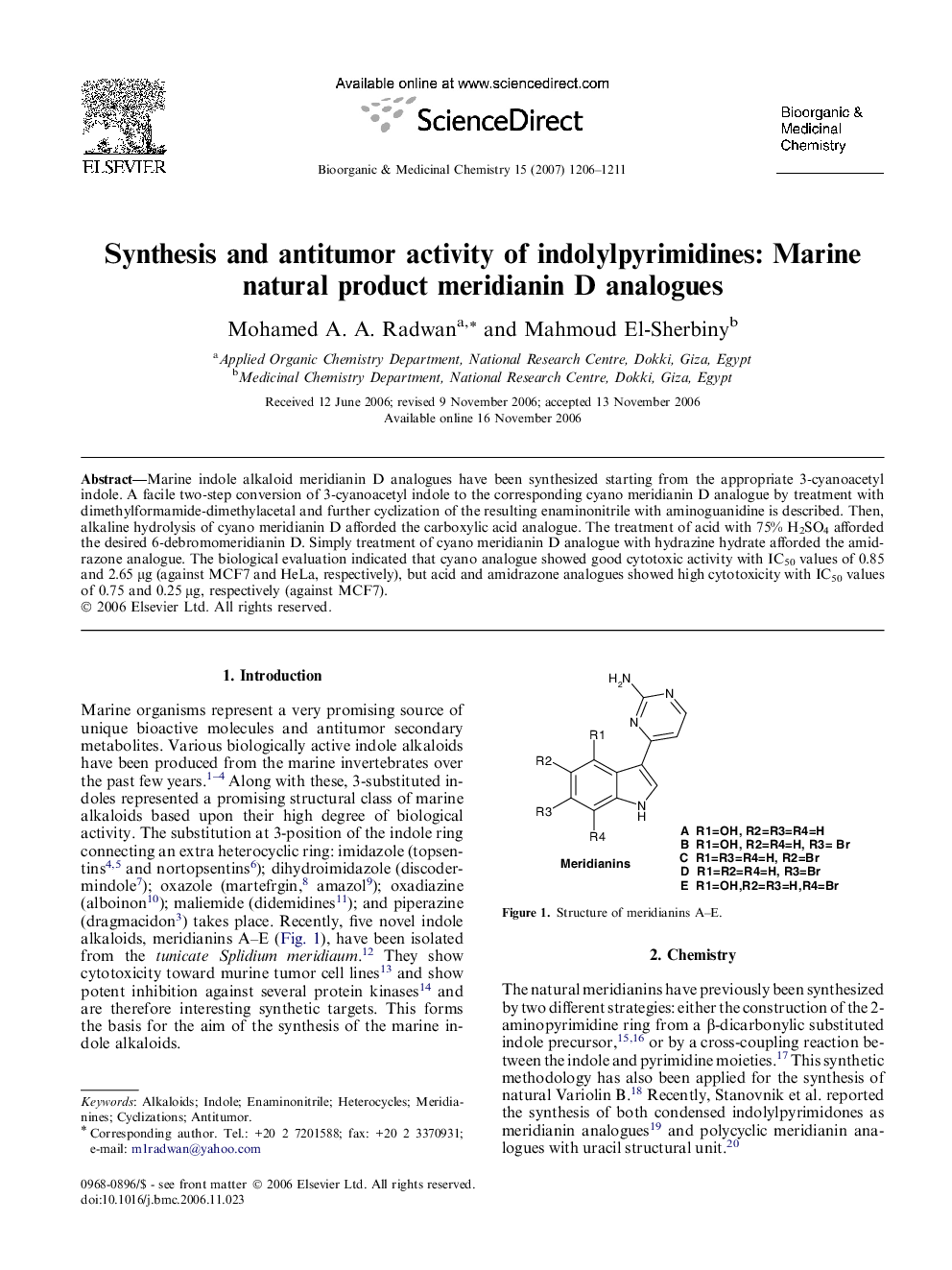
Marine indole alkaloid meridianin D analogues have been synthesized starting from the appropriate 3-cyanoacetyl indole. A facile two-step conversion of 3-cyanoacetyl indole to the corresponding cyano meridianin D analogue by treatment with dimethylformamide-dimethylacetal and further cyclization of the resulting enaminonitrile with aminoguanidine is described. Then, alkaline hydrolysis of cyano meridianin D afforded the carboxylic acid analogue. The treatment of acid with 75% H2SO4 afforded the desired 6-debromomeridianin D. Simply treatment of cyano meridianin D analogue with hydrazine hydrate afforded the amidrazone analogue. The biological evaluation indicated that cyano analogue showed good cytotoxic activity with IC50 values of 0.85 and 2.65 μg (against MCF7 and HeLa, respectively), but acid and amidrazone analogues showed high cytotoxicity with IC50 values of 0.75 and 0.25 μg, respectively (against MCF7).
Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry - Volume 15, Issue 3, 1 February 2007, Pages 1206–1211