| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1388015 | 982753 | 2010 | 12 صفحه PDF | دانلود رایگان |
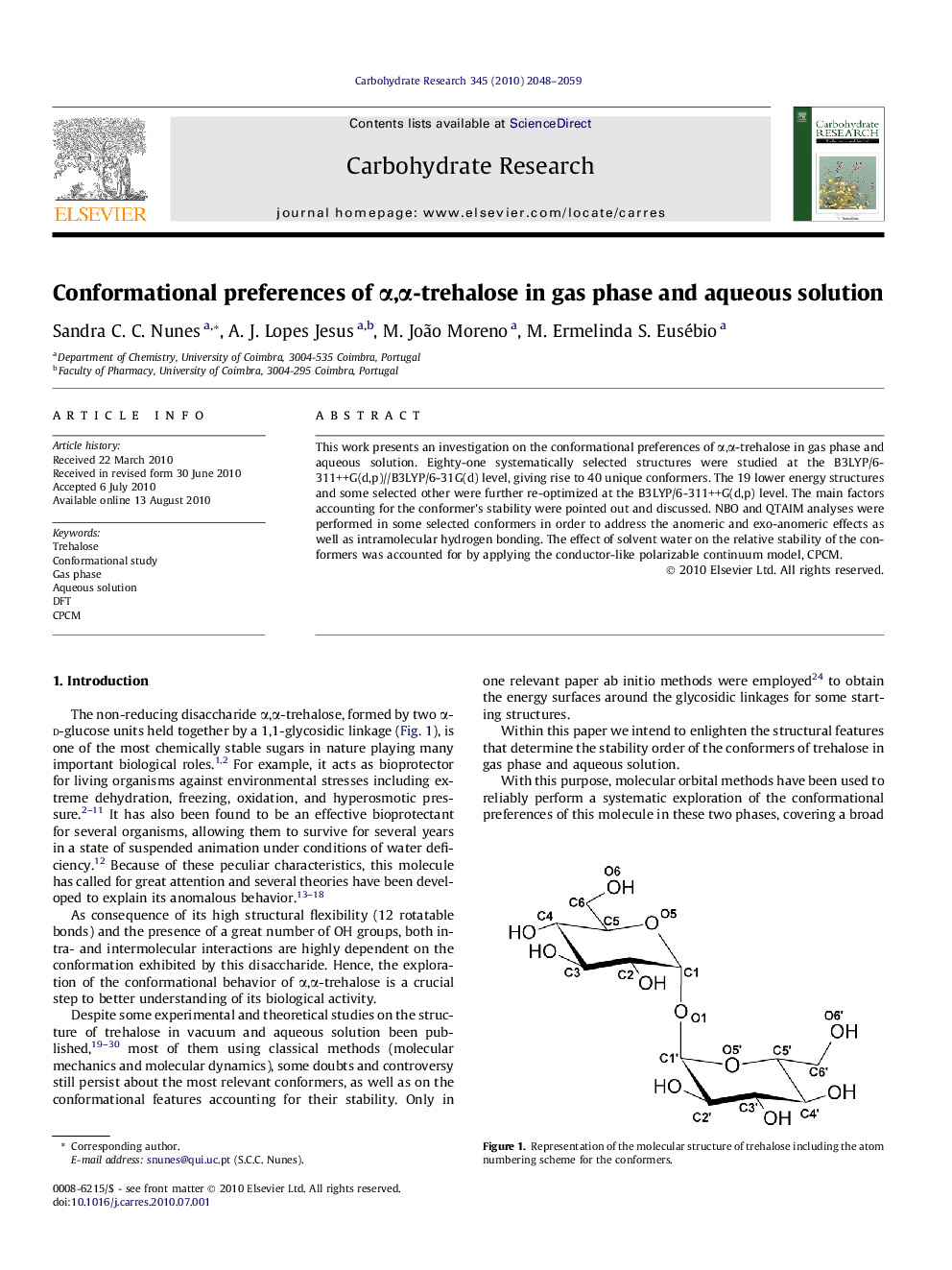
This work presents an investigation on the conformational preferences of α,α-trehalose in gas phase and aqueous solution. Eighty-one systematically selected structures were studied at the B3LYP/6-311++G(d,p)//B3LYP/6-31G(d) level, giving rise to 40 unique conformers. The 19 lower energy structures and some selected other were further re-optimized at the B3LYP/6-311++G(d,p) level. The main factors accounting for the conformer’s stability were pointed out and discussed. NBO and QTAIM analyses were performed in some selected conformers in order to address the anomeric and exo-anomeric effects as well as intramolecular hydrogen bonding. The effect of solvent water on the relative stability of the conformers was accounted for by applying the conductor-like polarizable continuum model, CPCM.
Figure optionsDownload as PowerPoint slide
Journal: Carbohydrate Research - Volume 345, Issue 14, 23 September 2010, Pages 2048–2059