| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1402290 | 1501742 | 2014 | 10 صفحه PDF | دانلود رایگان |
• Copper complexes containing amino acids and oxime have been characterized.
• A distorted octahedral geometry has been conjectured from DFT calculations.
• The electrochemical behavior was determined by cyclic voltammetry.
• The complexes show a good antifungal activity.
• Antioxidant activity for these complexes has been evaluated.
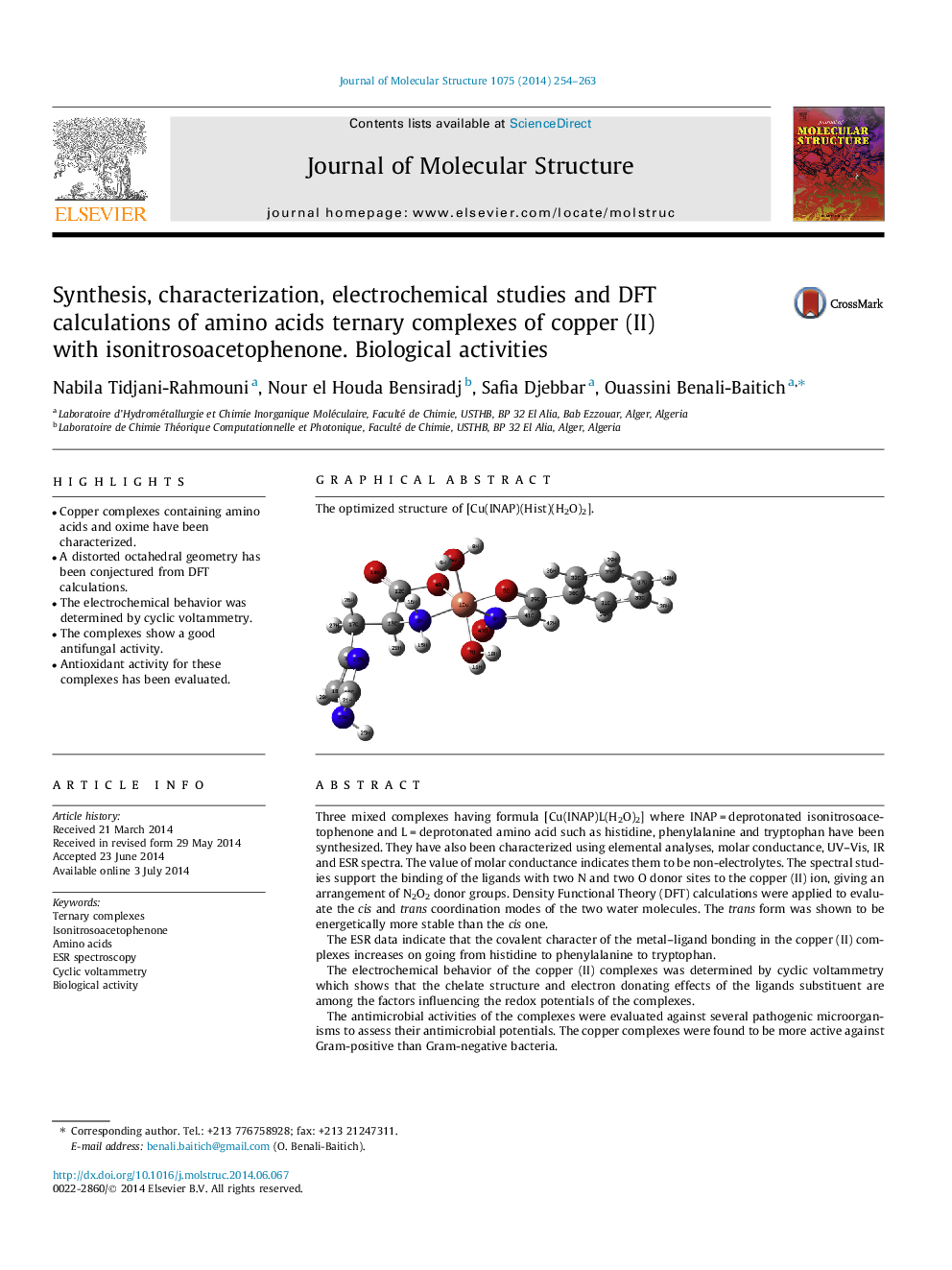
Three mixed complexes having formula [Cu(INAP)L(H2O)2] where INAP = deprotonated isonitrosoacetophenone and L = deprotonated amino acid such as histidine, phenylalanine and tryptophan have been synthesized. They have also been characterized using elemental analyses, molar conductance, UV–Vis, IR and ESR spectra. The value of molar conductance indicates them to be non-electrolytes. The spectral studies support the binding of the ligands with two N and two O donor sites to the copper (II) ion, giving an arrangement of N2O2 donor groups. Density Functional Theory (DFT) calculations were applied to evaluate the cis and trans coordination modes of the two water molecules. The trans form was shown to be energetically more stable than the cis one.The ESR data indicate that the covalent character of the metal–ligand bonding in the copper (II) complexes increases on going from histidine to phenylalanine to tryptophan.The electrochemical behavior of the copper (II) complexes was determined by cyclic voltammetry which shows that the chelate structure and electron donating effects of the ligands substituent are among the factors influencing the redox potentials of the complexes.The antimicrobial activities of the complexes were evaluated against several pathogenic microorganisms to assess their antimicrobial potentials. The copper complexes were found to be more active against Gram-positive than Gram-negative bacteria.Furthermore, the antioxidant efficiencies of the metal complexes were determined by 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity. The antioxidant activity of the complexes indicates their moderate scavenging activity against the radical DPPH.
The optimized structure of [Cu(INAP)(Hist)(H2O)2].Figure optionsDownload as PowerPoint slide
Journal: Journal of Molecular Structure - Volume 1075, 5 October 2014, Pages 254–263