| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1402298 | 1501742 | 2014 | 10 صفحه PDF | دانلود رایگان |
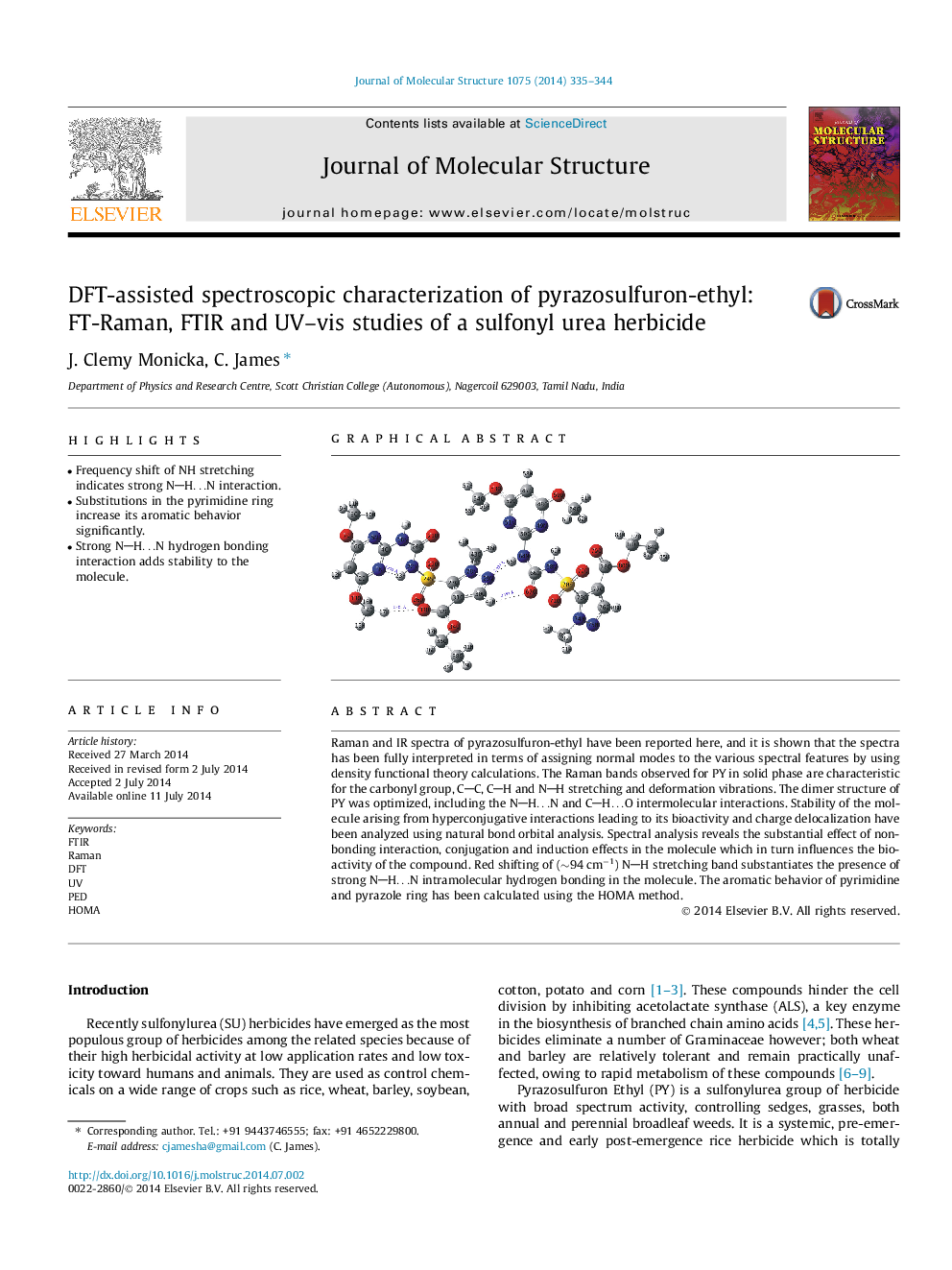
• Frequency shift of NH stretching indicates strong NH…N interaction.
• Substitutions in the pyrimidine ring increase its aromatic behavior significantly.
• Strong NH…N hydrogen bonding interaction adds stability to the molecule.
Raman and IR spectra of pyrazosulfuron-ethyl have been reported here, and it is shown that the spectra has been fully interpreted in terms of assigning normal modes to the various spectral features by using density functional theory calculations. The Raman bands observed for PY in solid phase are characteristic for the carbonyl group, CC, CH and NH stretching and deformation vibrations. The dimer structure of PY was optimized, including the NH…N and CH…O intermolecular interactions. Stability of the molecule arising from hyperconjugative interactions leading to its bioactivity and charge delocalization have been analyzed using natural bond orbital analysis. Spectral analysis reveals the substantial effect of non-bonding interaction, conjugation and induction effects in the molecule which in turn influences the bioactivity of the compound. Red shifting of (∼94 cm−1) NH stretching band substantiates the presence of strong NH…N intramolecular hydrogen bonding in the molecule. The aromatic behavior of pyrimidine and pyrazole ring has been calculated using the HOMA method.
Figure optionsDownload as PowerPoint slide
Journal: Journal of Molecular Structure - Volume 1075, 5 October 2014, Pages 335–344