| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1402867 | 1501762 | 2013 | 8 صفحه PDF | دانلود رایگان |
• Stereospecific synthesis of novel adenine isonucleosides analogous.
• Determination of crystal structures and its crystals packing features.
• Tetrahydropyran diastereoselectively prepared by Prins cyclization reaction.
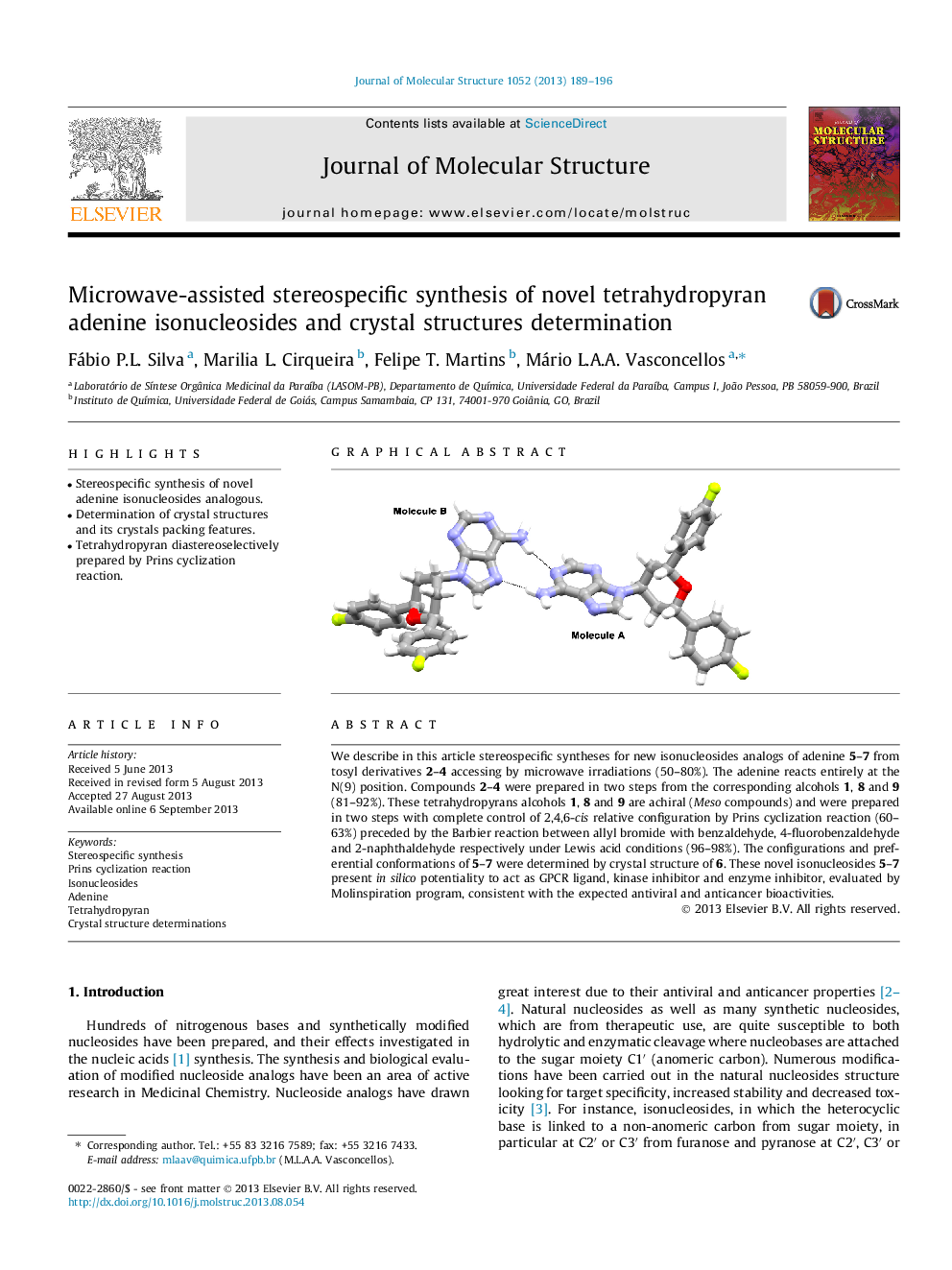
We describe in this article stereospecific syntheses for new isonucleosides analogs of adenine 5–7 from tosyl derivatives 2–4 accessing by microwave irradiations (50–80%). The adenine reacts entirely at the N(9) position. Compounds 2–4 were prepared in two steps from the corresponding alcohols 1, 8 and 9 (81–92%). These tetrahydropyrans alcohols 1, 8 and 9 are achiral (Meso compounds) and were prepared in two steps with complete control of 2,4,6-cis relative configuration by Prins cyclization reaction (60–63%) preceded by the Barbier reaction between allyl bromide with benzaldehyde, 4-fluorobenzaldehyde and 2-naphthaldehyde respectively under Lewis acid conditions (96–98%). The configurations and preferential conformations of 5–7 were determined by crystal structure of 6. These novel isonucleosides 5–7 present in silico potentiality to act as GPCR ligand, kinase inhibitor and enzyme inhibitor, evaluated by Molinspiration program, consistent with the expected antiviral and anticancer bioactivities.
Figure optionsDownload as PowerPoint slide
Journal: Journal of Molecular Structure - Volume 1052, 25 November 2013, Pages 189–196