| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1404851 | 1501724 | 2015 | 12 صفحه PDF | دانلود رایگان |
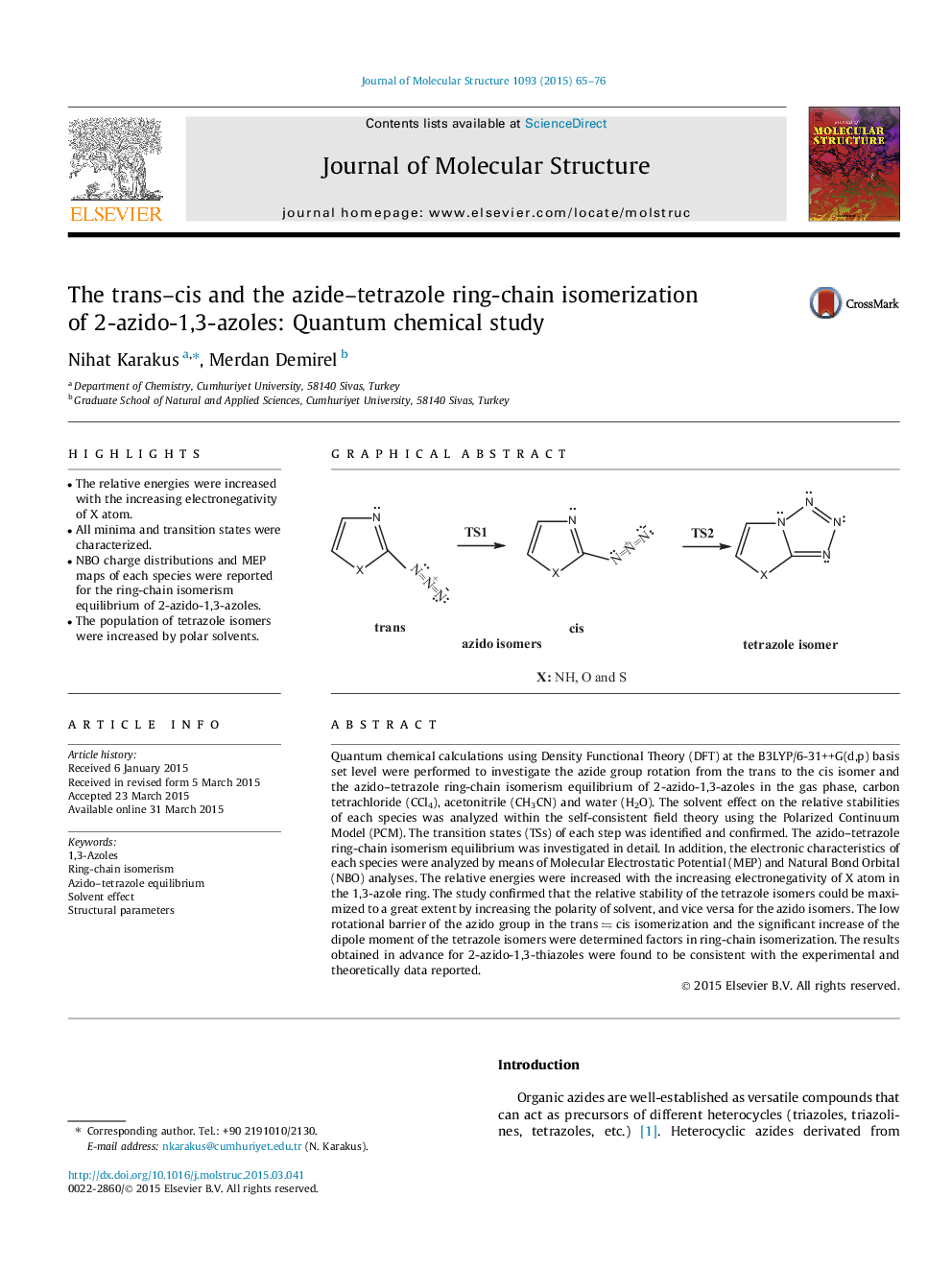
• The relative energies were increased with the increasing electronegativity of X atom.
• All minima and transition states were characterized.
• NBO charge distributions and MEP maps of each species were reported for the ring-chain isomerism equilibrium of 2-azido-1,3-azoles.
• The population of tetrazole isomers were increased by polar solvents.
Quantum chemical calculations using Density Functional Theory (DFT) at the B3LYP/6-31++G(d,p) basis set level were performed to investigate the azide group rotation from the trans to the cis isomer and the azido–tetrazole ring-chain isomerism equilibrium of 2-azido-1,3-azoles in the gas phase, carbon tetrachloride (CCl4), acetonitrile (CH3CN) and water (H2O). The solvent effect on the relative stabilities of each species was analyzed within the self-consistent field theory using the Polarized Continuum Model (PCM). The transition states (TSs) of each step was identified and confirmed. The azido–tetrazole ring-chain isomerism equilibrium was investigated in detail. In addition, the electronic characteristics of each species were analyzed by means of Molecular Electrostatic Potential (MEP) and Natural Bond Orbital (NBO) analyses. The relative energies were increased with the increasing electronegativity of X atom in the 1,3-azole ring. The study confirmed that the relative stability of the tetrazole isomers could be maximized to a great extent by increasing the polarity of solvent, and vice versa for the azido isomers. The low rotational barrier of the azido group in the trans ⇋ cis isomerization and the significant increase of the dipole moment of the tetrazole isomers were determined factors in ring-chain isomerization. The results obtained in advance for 2-azido-1,3-thiazoles were found to be consistent with the experimental and theoretically data reported.
Figure optionsDownload as PowerPoint slide
Journal: Journal of Molecular Structure - Volume 1093, 5 August 2015, Pages 65–76