| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1405021 | 1501745 | 2014 | 6 صفحه PDF | دانلود رایگان |
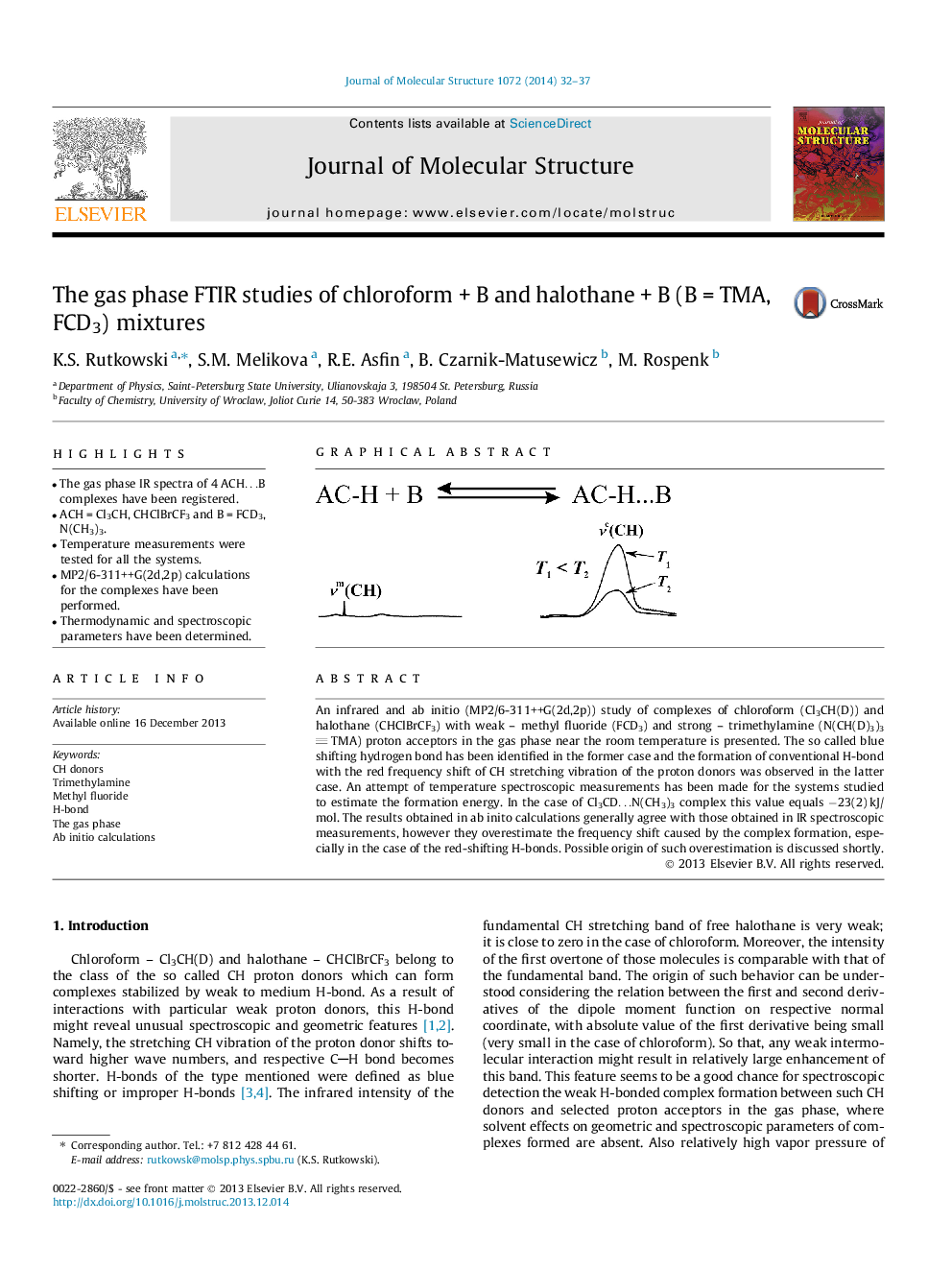
• The gas phase IR spectra of 4 ACH…B complexes have been registered.
• ACH = Cl3CH, CHClBrCF3 and B = FCD3, N(CH3)3.
• Temperature measurements were tested for all the systems.
• MP2/6-311++G(2d,2p) calculations for the complexes have been performed.
• Thermodynamic and spectroscopic parameters have been determined.
An infrared and ab initio (MP2/6-311++G(2d,2p)) study of complexes of chloroform (Cl3CH(D)) and halothane (CHClBrCF3) with weak – methyl fluoride (FCD3) and strong – trimethylamine (N(CH(D)3)3 TMA) proton acceptors in the gas phase near the room temperature is presented. The so called blue shifting hydrogen bond has been identified in the former case and the formation of conventional H-bond with the red frequency shift of CH stretching vibration of the proton donors was observed in the latter case. An attempt of temperature spectroscopic measurements has been made for the systems studied to estimate the formation energy. In the case of Cl3CD…N(CH3)3 complex this value equals −23(2) kJ/mol. The results obtained in ab inito calculations generally agree with those obtained in IR spectroscopic measurements, however they overestimate the frequency shift caused by the complex formation, especially in the case of the red-shifting H-bonds. Possible origin of such overestimation is discussed shortly.
Figure optionsDownload as PowerPoint slide
Journal: Journal of Molecular Structure - Volume 1072, 25 August 2014, Pages 32–37