| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1405559 | 1501749 | 2014 | 9 صفحه PDF | دانلود رایگان |
• Proteins with partially unfolded conformations were made in silico.
• TNS was docked against different conformations of the proteins.
• Dynamic simulation of protein–TNS complexes were carried out.
• Resultant conformations were analyzed.
• TNS fluorescence due to hydrophobic environment and reduced solvent accessibility.
• Binding orientation and binding ability has less contribution to fluorescence.
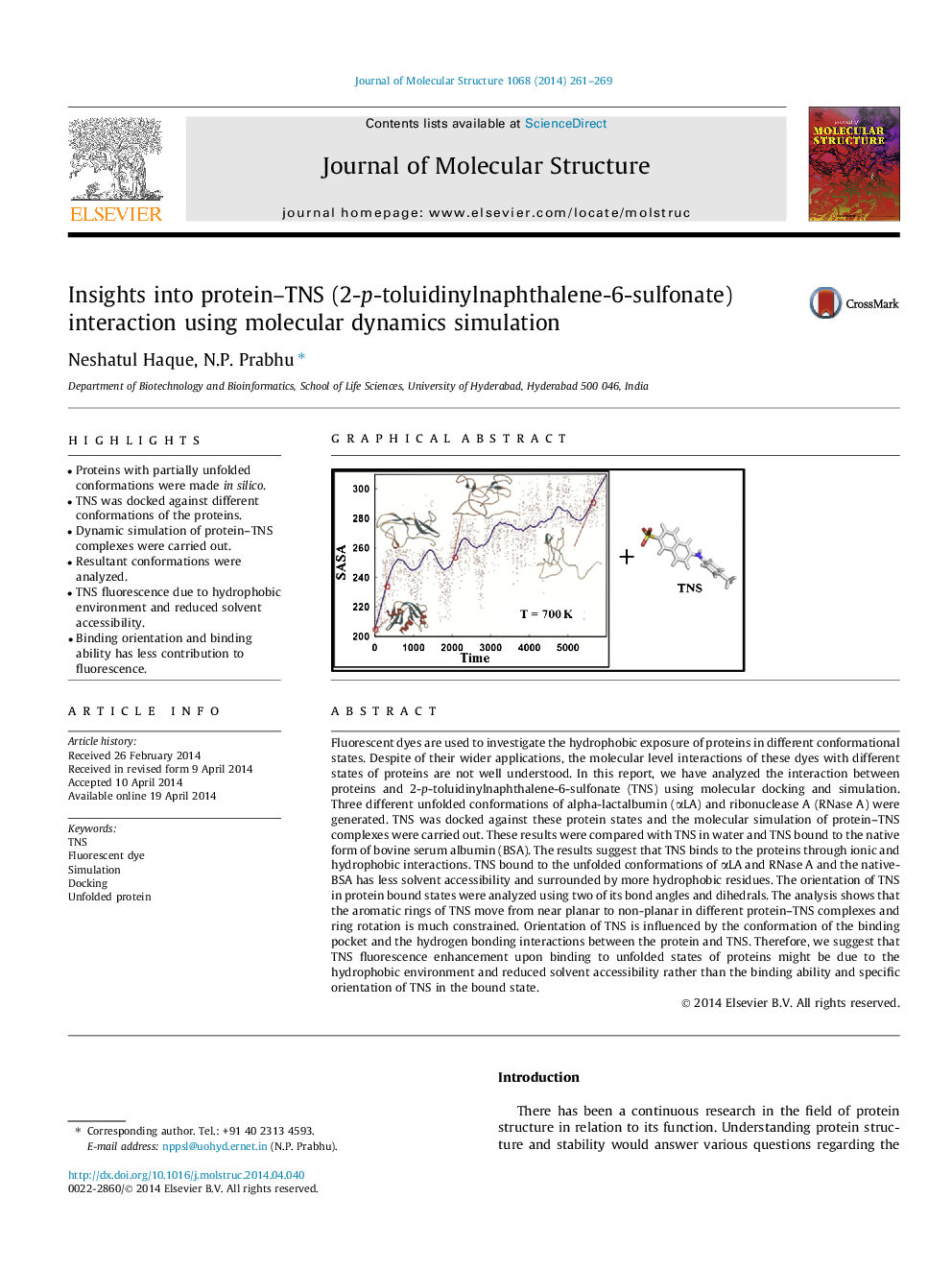
Fluorescent dyes are used to investigate the hydrophobic exposure of proteins in different conformational states. Despite of their wider applications, the molecular level interactions of these dyes with different states of proteins are not well understood. In this report, we have analyzed the interaction between proteins and 2-p-toluidinylnaphthalene-6-sulfonate (TNS) using molecular docking and simulation. Three different unfolded conformations of alpha-lactalbumin (αLA) and ribonuclease A (RNase A) were generated. TNS was docked against these protein states and the molecular simulation of protein–TNS complexes were carried out. These results were compared with TNS in water and TNS bound to the native form of bovine serum albumin (BSA). The results suggest that TNS binds to the proteins through ionic and hydrophobic interactions. TNS bound to the unfolded conformations of αLA and RNase A and the native-BSA has less solvent accessibility and surrounded by more hydrophobic residues. The orientation of TNS in protein bound states were analyzed using two of its bond angles and dihedrals. The analysis shows that the aromatic rings of TNS move from near planar to non-planar in different protein–TNS complexes and ring rotation is much constrained. Orientation of TNS is influenced by the conformation of the binding pocket and the hydrogen bonding interactions between the protein and TNS. Therefore, we suggest that TNS fluorescence enhancement upon binding to unfolded states of proteins might be due to the hydrophobic environment and reduced solvent accessibility rather than the binding ability and specific orientation of TNS in the bound state.
Figure optionsDownload as PowerPoint slide
Journal: Journal of Molecular Structure - Volume 1068, 25 June 2014, Pages 261–269