| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1405693 | 1501757 | 2014 | 10 صفحه PDF | دانلود رایگان |
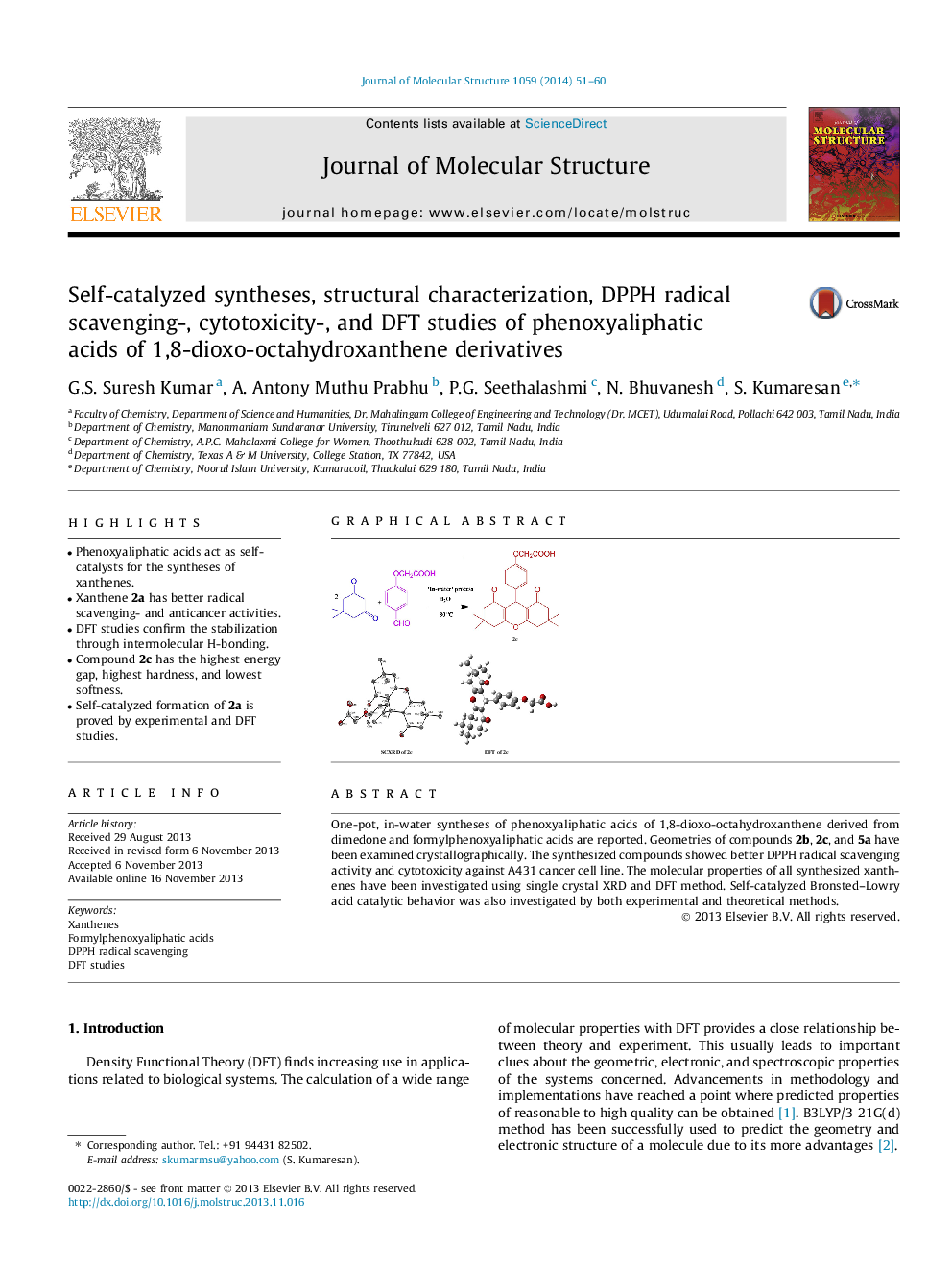
• Phenoxyaliphatic acids act as self-catalysts for the syntheses of xanthenes.
• Xanthene 2a has better radical scavenging- and anticancer activities.
• DFT studies confirm the stabilization through intermolecular H-bonding.
• Compound 2c has the highest energy gap, highest hardness, and lowest softness.
• Self-catalyzed formation of 2a is proved by experimental and DFT studies.
One-pot, in-water syntheses of phenoxyaliphatic acids of 1,8-dioxo-octahydroxanthene derived from dimedone and formylphenoxyaliphatic acids are reported. Geometries of compounds 2b, 2c, and 5a have been examined crystallographically. The synthesized compounds showed better DPPH radical scavenging activity and cytotoxicity against A431 cancer cell line. The molecular properties of all synthesized xanthenes have been investigated using single crystal XRD and DFT method. Self-catalyzed Bronsted–Lowry acid catalytic behavior was also investigated by both experimental and theoretical methods.
Figure optionsDownload as PowerPoint slide
Journal: Journal of Molecular Structure - Volume 1059, 5 February 2014, Pages 51–60