| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1408146 | 1501723 | 2015 | 10 صفحه PDF | دانلود رایگان |
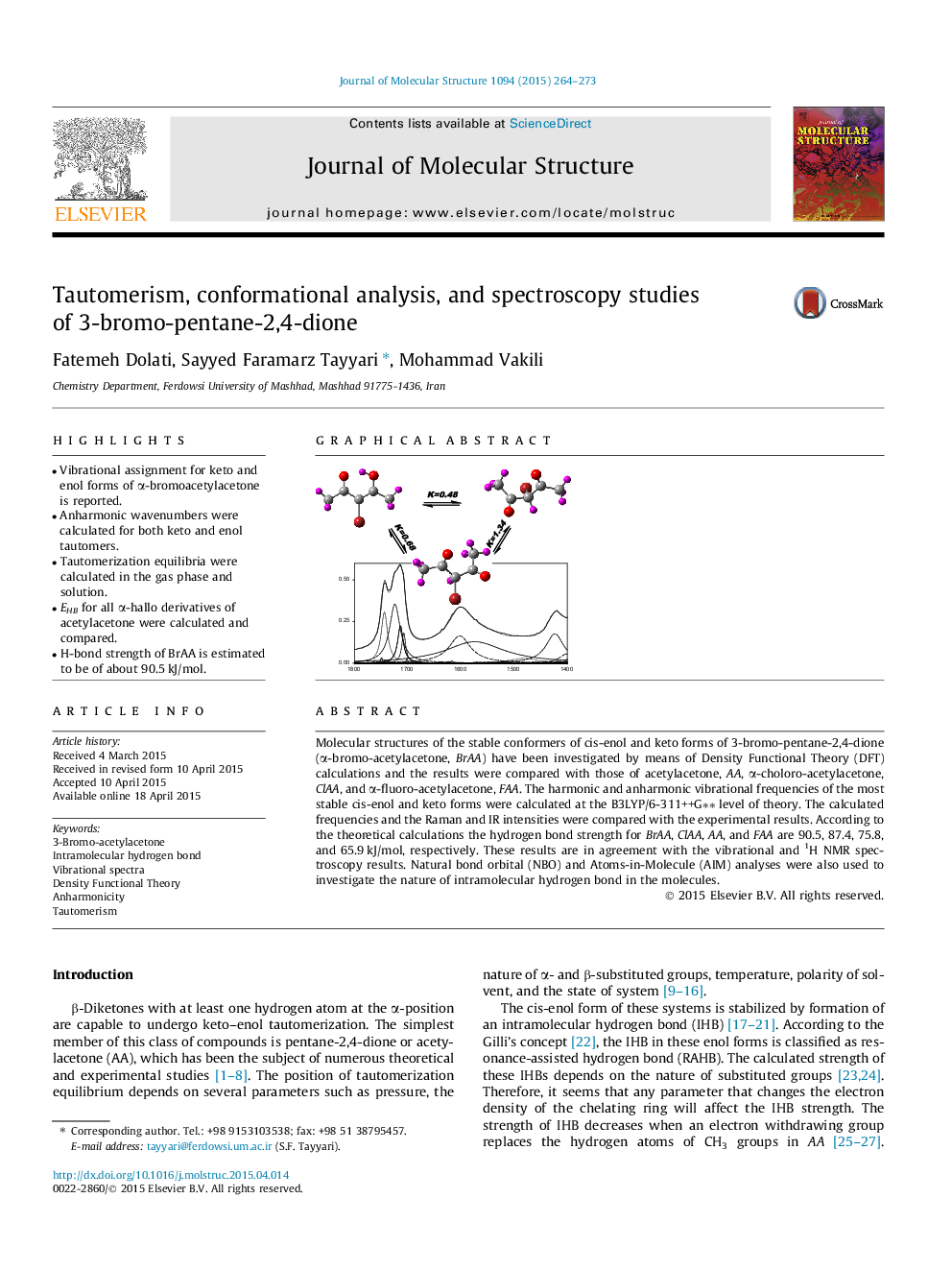
• Vibrational assignment for keto and enol forms of α-bromoacetylacetone is reported.
• Anharmonic wavenumbers were calculated for both keto and enol tautomers.
• Tautomerization equilibria were calculated in the gas phase and solution.
• EHB for all α-hallo derivatives of acetylacetone were calculated and compared.
• H-bond strength of BrAA is estimated to be of about 90.5 kJ/mol.
Molecular structures of the stable conformers of cis-enol and keto forms of 3-bromo-pentane-2,4-dione (α-bromo-acetylacetone, BrAA) have been investigated by means of Density Functional Theory (DFT) calculations and the results were compared with those of acetylacetone, AA, α-choloro-acetylacetone, ClAA, and α-fluoro-acetylacetone, FAA. The harmonic and anharmonic vibrational frequencies of the most stable cis-enol and keto forms were calculated at the B3LYP/6-311++G∗∗ level of theory. The calculated frequencies and the Raman and IR intensities were compared with the experimental results. According to the theoretical calculations the hydrogen bond strength for BrAA, ClAA, AA, and FAA are 90.5, 87.4, 75.8, and 65.9 kJ/mol, respectively. These results are in agreement with the vibrational and 1H NMR spectroscopy results. Natural bond orbital (NBO) and Atoms-in-Molecule (AIM) analyses were also used to investigate the nature of intramolecular hydrogen bond in the molecules.
Figure optionsDownload as PowerPoint slide
Journal: Journal of Molecular Structure - Volume 1094, 15 August 2015, Pages 264–273