| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1408148 | 1501723 | 2015 | 11 صفحه PDF | دانلود رایگان |
• Acyl thiourea showed good anti-oxidant and anti-haemolytic activities.
• Molecular docking for biological evaluation.
• Structure was confirmed by X-ray crystallography.
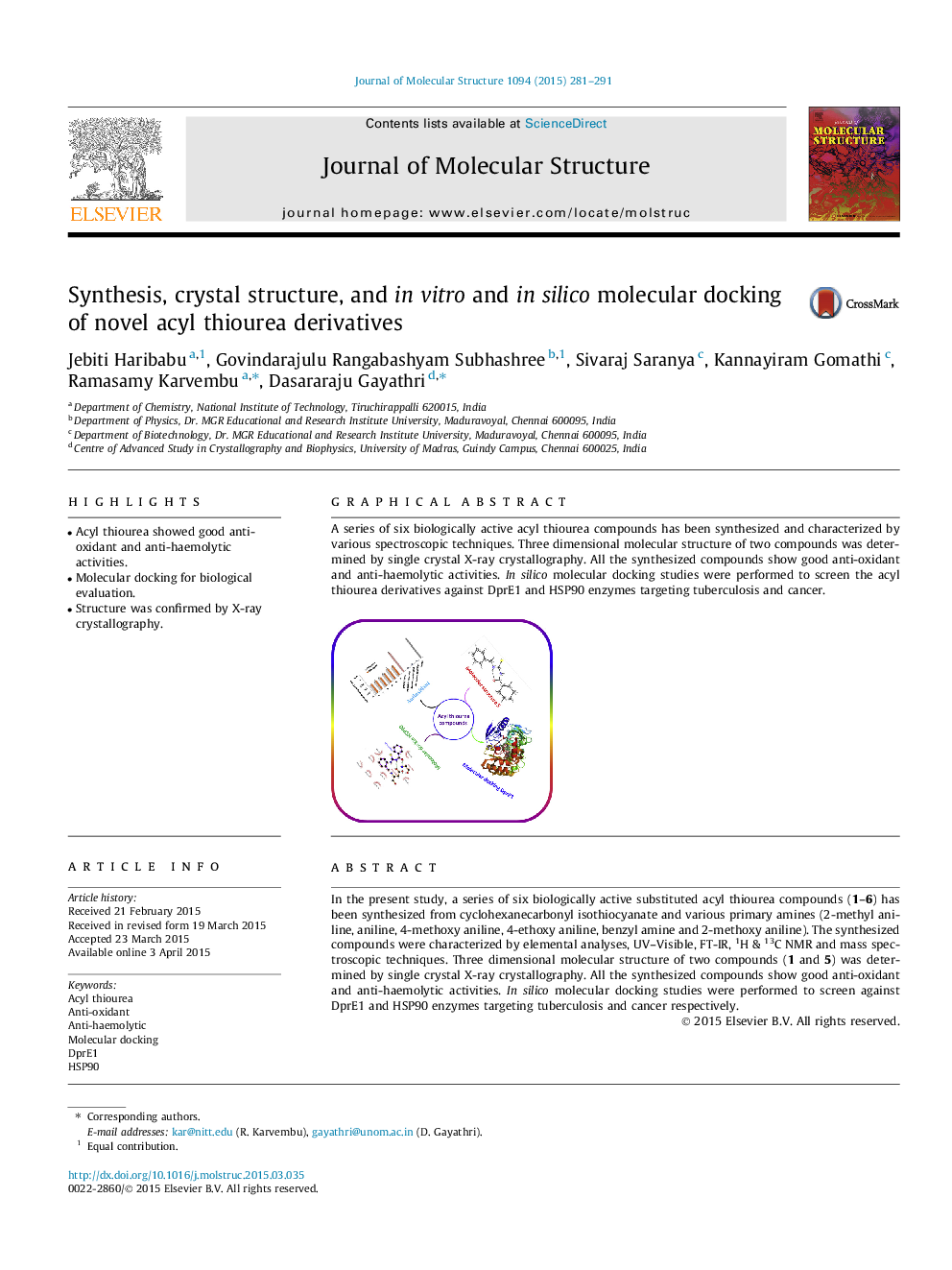
In the present study, a series of six biologically active substituted acyl thiourea compounds (1–6) has been synthesized from cyclohexanecarbonyl isothiocyanate and various primary amines (2-methyl aniline, aniline, 4-methoxy aniline, 4-ethoxy aniline, benzyl amine and 2-methoxy aniline). The synthesized compounds were characterized by elemental analyses, UV–Visible, FT-IR, 1H & 13C NMR and mass spectroscopic techniques. Three dimensional molecular structure of two compounds (1 and 5) was determined by single crystal X-ray crystallography. All the synthesized compounds show good anti-oxidant and anti-haemolytic activities. In silico molecular docking studies were performed to screen against DprE1 and HSP90 enzymes targeting tuberculosis and cancer respectively.
A series of six biologically active acyl thiourea compounds has been synthesized and characterized by various spectroscopic techniques. Three dimensional molecular structure of two compounds was determined by single crystal X-ray crystallography. All the synthesized compounds show good anti-oxidant and anti-haemolytic activities. In silico molecular docking studies were performed to screen the acyl thiourea derivatives against DprE1 and HSP90 enzymes targeting tuberculosis and cancer.Figure optionsDownload as PowerPoint slide
Journal: Journal of Molecular Structure - Volume 1094, 15 August 2015, Pages 281–291