| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1409295 | 1501746 | 2014 | 6 صفحه PDF | دانلود رایگان |
• The influence of temperature and light irradiation of aqueous solutions riboflavin was studied.
• The riboflavin has undergone destruction depending on the concentration and magnitude of temperature effects.
• The presence of various acids and metal ions in the solution leads to increased photostability.
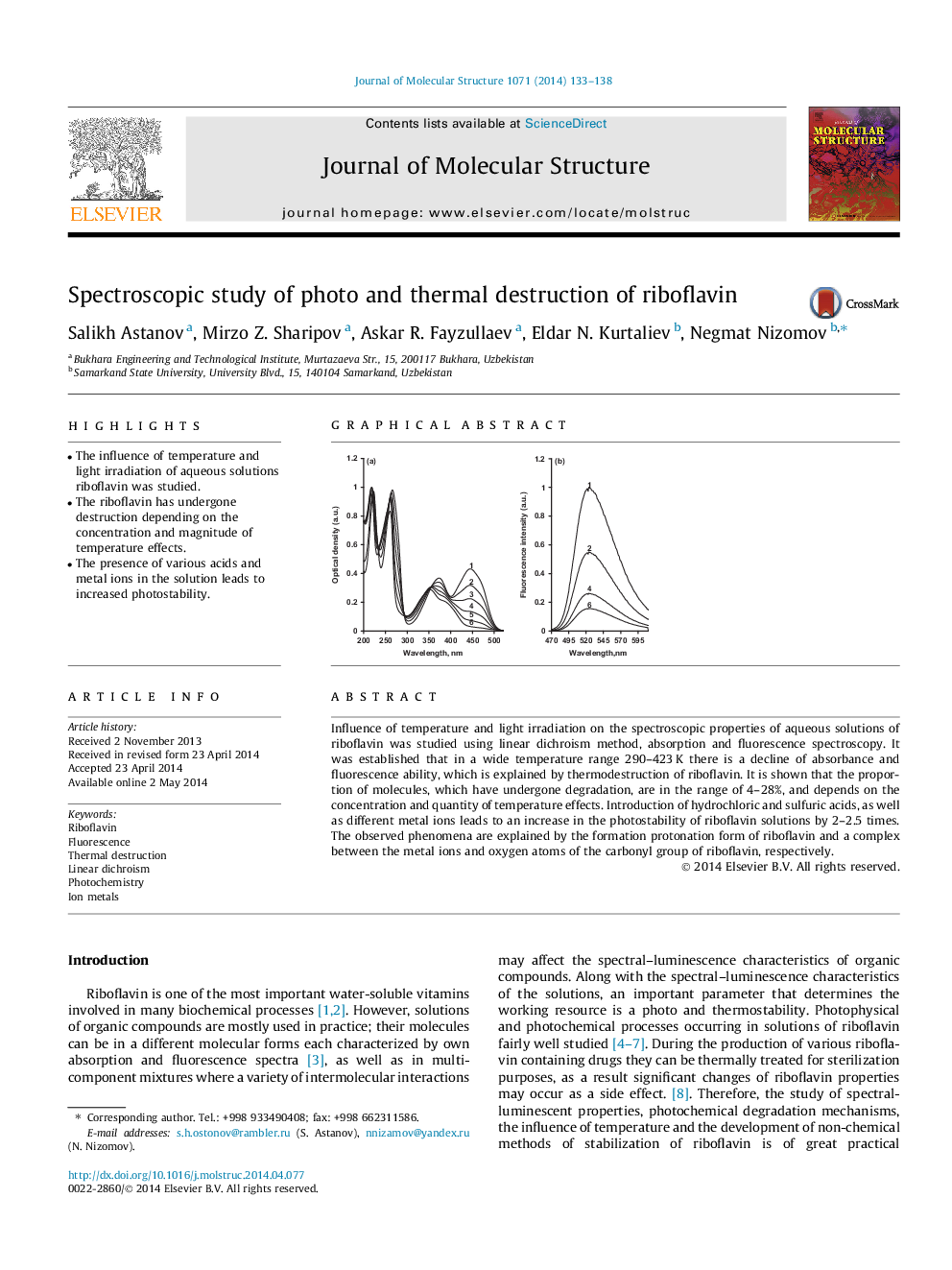
Influence of temperature and light irradiation on the spectroscopic properties of aqueous solutions of riboflavin was studied using linear dichroism method, absorption and fluorescence spectroscopy. It was established that in a wide temperature range 290–423 K there is a decline of absorbance and fluorescence ability, which is explained by thermodestruction of riboflavin. It is shown that the proportion of molecules, which have undergone degradation, are in the range of 4–28%, and depends on the concentration and quantity of temperature effects. Introduction of hydrochloric and sulfuric acids, as well as different metal ions leads to an increase in the photostability of riboflavin solutions by 2–2.5 times. The observed phenomena are explained by the formation protonation form of riboflavin and a complex between the metal ions and oxygen atoms of the carbonyl group of riboflavin, respectively.
The absorption (a) and fluorescence (b) spectra of aqueous solutions of riboflavin exposed to irradiation by light: 1–0, 2–22, 3–47, 4–74, 5–100, 6–160 min.Figure optionsDownload as PowerPoint slide
Journal: Journal of Molecular Structure - Volume 1071, 5 August 2014, Pages 133–138