| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 146044 | 456356 | 2016 | 8 صفحه PDF | دانلود رایگان |
• Diclofenac sodium can be adsorbed effectively over UiO-66-SO3H.
• The adsorption capacity and rate over UiO-66-SO3H are remarkably high.
• The UiO-66-SO3H can be used especially at low concentration of diclofenac sodium.
• The adsorption might be explained with electrostatic and acid–base interactions.
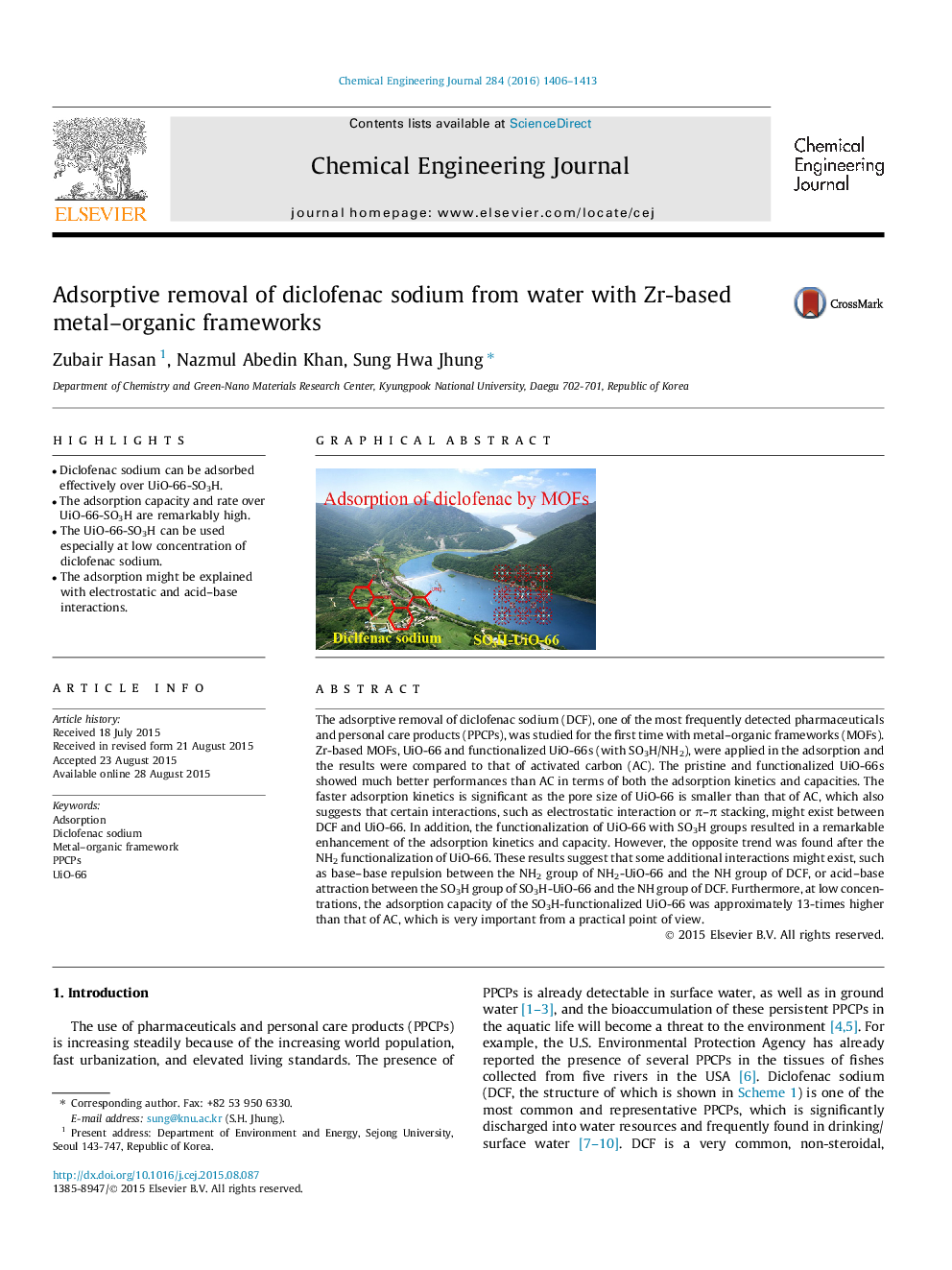
The adsorptive removal of diclofenac sodium (DCF), one of the most frequently detected pharmaceuticals and personal care products (PPCPs), was studied for the first time with metal–organic frameworks (MOFs). Zr-based MOFs, UiO-66 and functionalized UiO-66s (with SO3H/NH2), were applied in the adsorption and the results were compared to that of activated carbon (AC). The pristine and functionalized UiO-66s showed much better performances than AC in terms of both the adsorption kinetics and capacities. The faster adsorption kinetics is significant as the pore size of UiO-66 is smaller than that of AC, which also suggests that certain interactions, such as electrostatic interaction or π–π stacking, might exist between DCF and UiO-66. In addition, the functionalization of UiO-66 with SO3H groups resulted in a remarkable enhancement of the adsorption kinetics and capacity. However, the opposite trend was found after the NH2 functionalization of UiO-66. These results suggest that some additional interactions might exist, such as base–base repulsion between the NH2 group of NH2-UiO-66 and the NH group of DCF, or acid–base attraction between the SO3H group of SO3H-UiO-66 and the NH group of DCF. Furthermore, at low concentrations, the adsorption capacity of the SO3H-functionalized UiO-66 was approximately 13-times higher than that of AC, which is very important from a practical point of view.
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 284, 15 January 2016, Pages 1406–1413