| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 146375 | 456368 | 2015 | 8 صفحه PDF | دانلود رایگان |
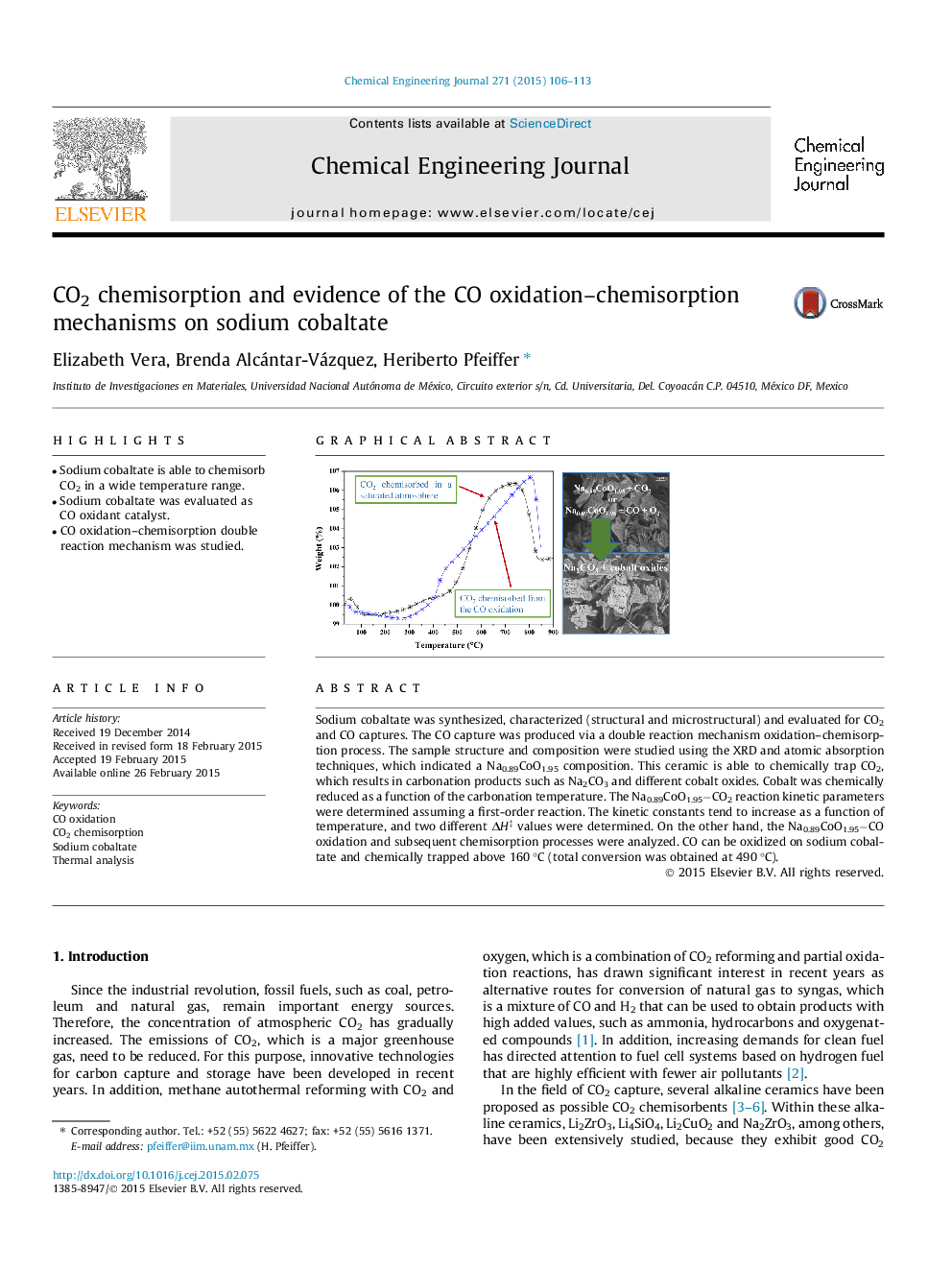
• Sodium cobaltate is able to chemisorb CO2 in a wide temperature range.
• Sodium cobaltate was evaluated as CO oxidant catalyst.
• CO oxidation–chemisorption double reaction mechanism was studied.
Sodium cobaltate was synthesized, characterized (structural and microstructural) and evaluated for CO2 and CO captures. The CO capture was produced via a double reaction mechanism oxidation–chemisorption process. The sample structure and composition were studied using the XRD and atomic absorption techniques, which indicated a Na0.89CoO1.95 composition. This ceramic is able to chemically trap CO2, which results in carbonation products such as Na2CO3 and different cobalt oxides. Cobalt was chemically reduced as a function of the carbonation temperature. The Na0.89CoO1.95−CO2 reaction kinetic parameters were determined assuming a first-order reaction. The kinetic constants tend to increase as a function of temperature, and two different ΔH‡ values were determined. On the other hand, the Na0.89CoO1.95−CO oxidation and subsequent chemisorption processes were analyzed. CO can be oxidized on sodium cobaltate and chemically trapped above 160 °C (total conversion was obtained at 490 °C).
Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 271, 1 July 2015, Pages 106–113