| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 216761 | 1426303 | 2009 | 7 صفحه PDF | دانلود رایگان |
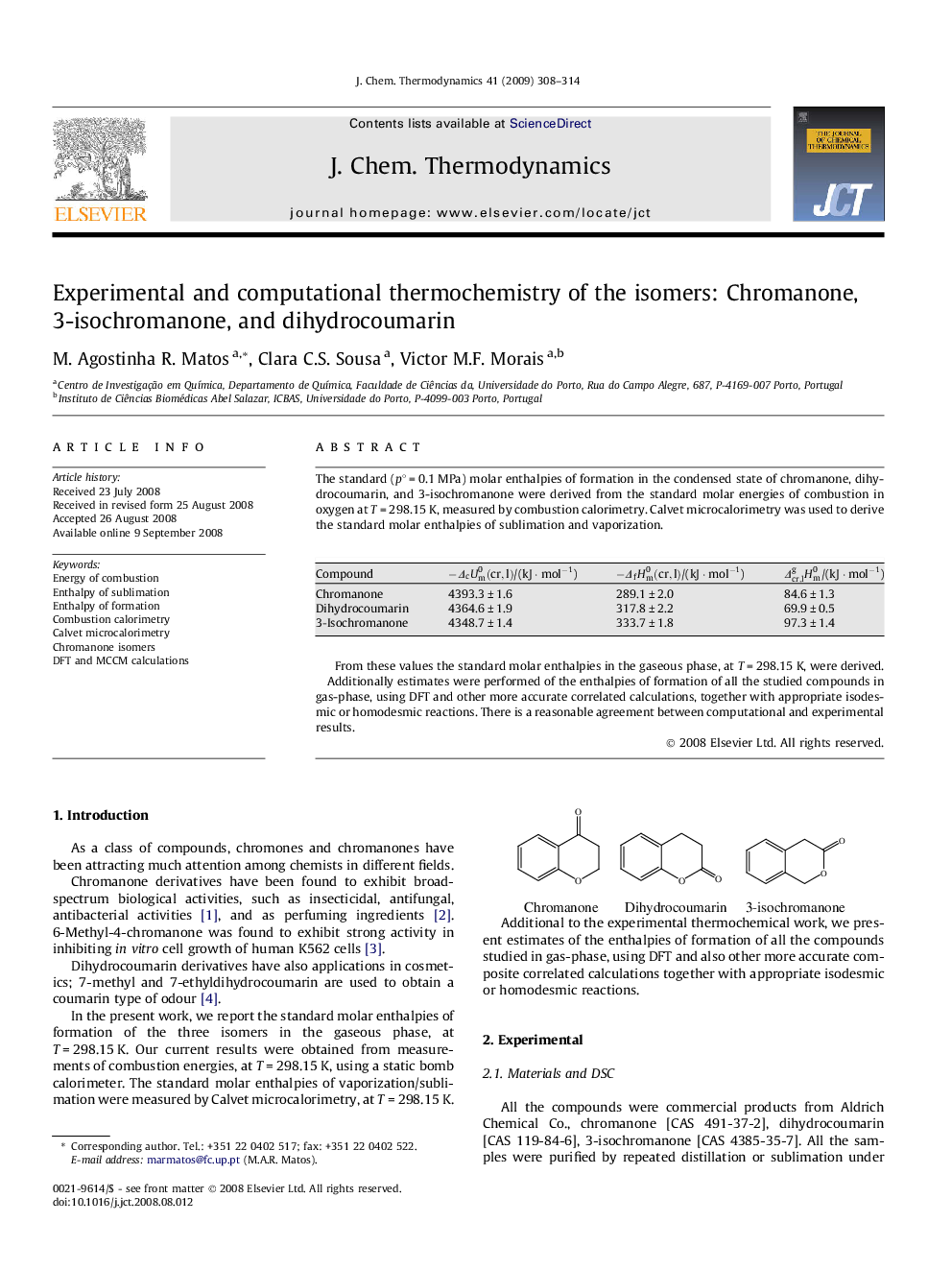
The standard (p∘ = 0.1 MPa) molar enthalpies of formation in the condensed state of chromanone, dihydrocoumarin, and 3-isochromanone were derived from the standard molar energies of combustion in oxygen at T = 298.15 K, measured by combustion calorimetry. Calvet microcalorimetry was used to derive the standard molar enthalpies of sublimation and vaporization.Compound-ΔcUm0(cr,l)/(kJ · mol−1)-ΔfHm0(cr,l)/(kJ · mol−1)Δcr,lgHm0/(kJ · mol−1)Chromanone4393.3 ± 1.6289.1 ± 2.084.6 ± 1.3Dihydrocoumarin4364.6 ± 1.9317.8 ± 2.269.9 ± 0.53-Isochromanone4348.7 ± 1.4333.7 ± 1.897.3 ± 1.4Full-size tableTable optionsView in workspaceDownload as CSVFrom these values the standard molar enthalpies in the gaseous phase, at T = 298.15 K, were derived.Additionally estimates were performed of the enthalpies of formation of all the studied compounds in gas-phase, using DFT and other more accurate correlated calculations, together with appropriate isodesmic or homodesmic reactions. There is a reasonable agreement between computational and experimental results.
Journal: The Journal of Chemical Thermodynamics - Volume 41, Issue 3, March 2009, Pages 308–314