| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 2184459 | 1095856 | 2013 | 9 صفحه PDF | دانلود رایگان |
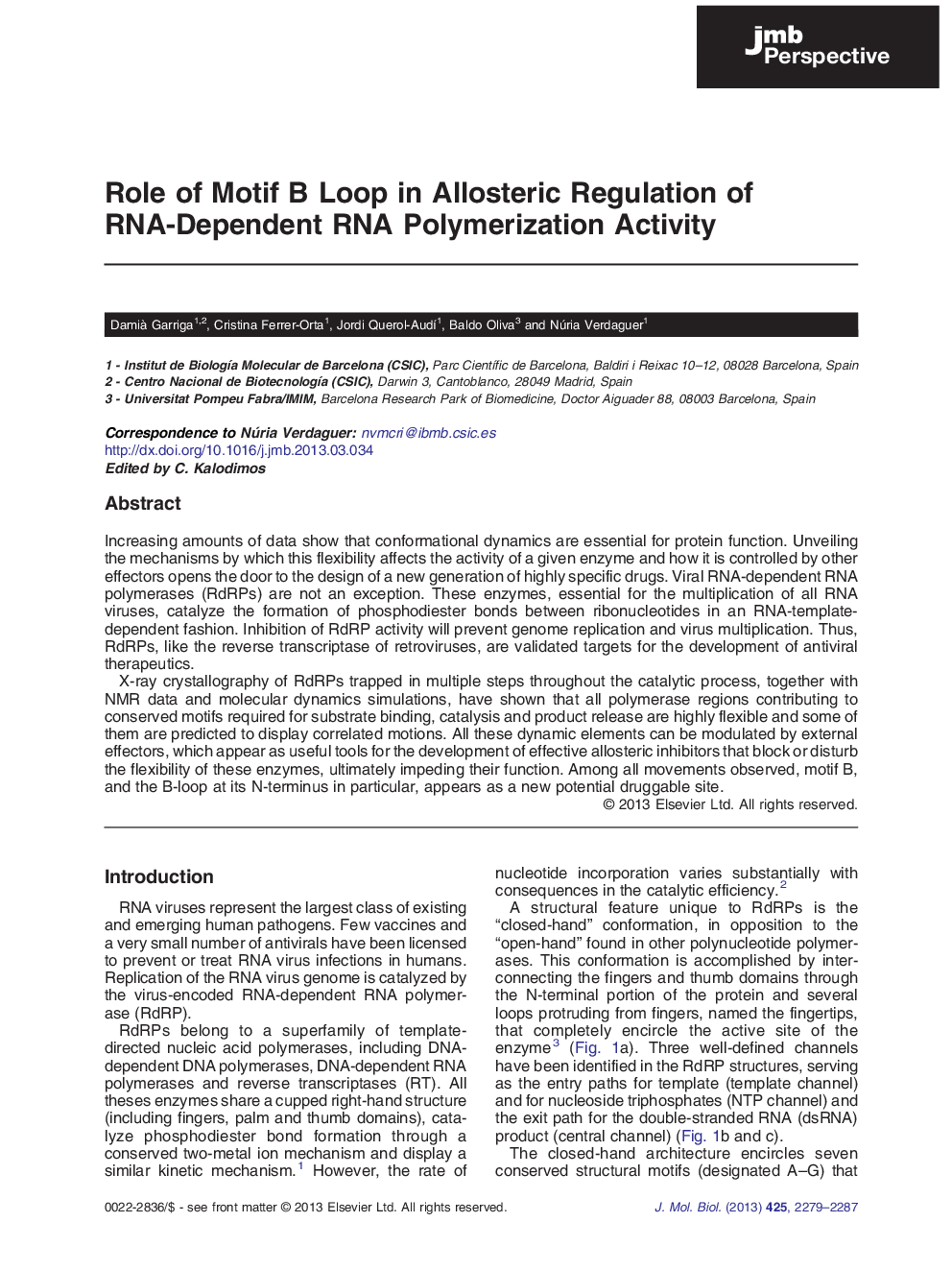
► Structures of RdRP catalytic complexes provide a glimpse into RNA polymerization.
► Compared X-ray and NMR structures, together with molecular dynamics, serve to find dynamic regions in RdRPs.
► Flexible elements of RdRPs are potential sites for antiviral drug discovery.
► B-loop is dynamic element in RdRPs that can be regulated by external factors.
Increasing amounts of data show that conformational dynamics are essential for protein function. Unveiling the mechanisms by which this flexibility affects the activity of a given enzyme and how it is controlled by other effectors opens the door to the design of a new generation of highly specific drugs. Viral RNA-dependent RNA polymerases (RdRPs) are not an exception. These enzymes, essential for the multiplication of all RNA viruses, catalyze the formation of phosphodiester bonds between ribonucleotides in an RNA-template-dependent fashion. Inhibition of RdRP activity will prevent genome replication and virus multiplication. Thus, RdRPs, like the reverse transcriptase of retroviruses, are validated targets for the development of antiviral therapeutics.X-ray crystallography of RdRPs trapped in multiple steps throughout the catalytic process, together with NMR data and molecular dynamics simulations, have shown that all polymerase regions contributing to conserved motifs required for substrate binding, catalysis and product release are highly flexible and some of them are predicted to display correlated motions. All these dynamic elements can be modulated by external effectors, which appear as useful tools for the development of effective allosteric inhibitors that block or disturb the flexibility of these enzymes, ultimately impeding their function. Among all movements observed, motif B, and the B-loop at its N-terminus in particular, appears as a new potential druggable site.
Graphical AbstractFigure optionsDownload high-quality image (186 K)Download as PowerPoint slide
Journal: Journal of Molecular Biology - Volume 425, Issue 13, 10 July 2013, Pages 2279–2287