| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 4407474 | 1618812 | 2016 | 8 صفحه PDF | دانلود رایگان |
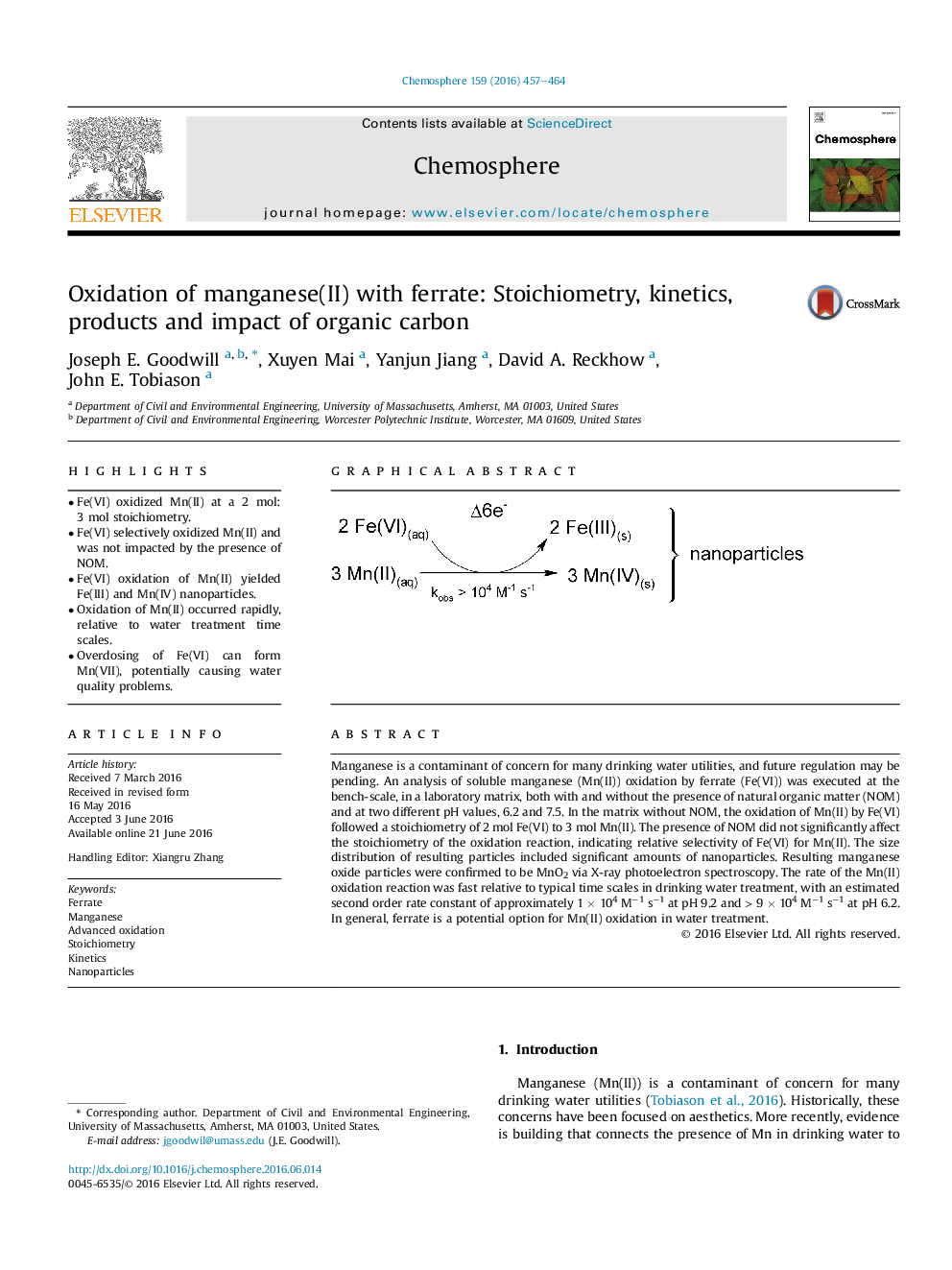
• Fe(VI) oxidized Mn(II) at a 2 mol: 3 mol stoichiometry.
• Fe(VI) selectively oxidized Mn(II) and was not impacted by the presence of NOM.
• Fe(VI) oxidation of Mn(II) yielded Fe(III) and Mn(IV) nanoparticles.
• Oxidation of Mn(II) occurred rapidly, relative to water treatment time scales.
• Overdosing of Fe(VI) can form Mn(VII), potentially causing water quality problems.
Manganese is a contaminant of concern for many drinking water utilities, and future regulation may be pending. An analysis of soluble manganese (Mn(II)) oxidation by ferrate (Fe(VI)) was executed at the bench-scale, in a laboratory matrix, both with and without the presence of natural organic matter (NOM) and at two different pH values, 6.2 and 7.5. In the matrix without NOM, the oxidation of Mn(II) by Fe(VI) followed a stoichiometry of 2 mol Fe(VI) to 3 mol Mn(II). The presence of NOM did not significantly affect the stoichiometry of the oxidation reaction, indicating relative selectivity of Fe(VI) for Mn(II). The size distribution of resulting particles included significant amounts of nanoparticles. Resulting manganese oxide particles were confirmed to be MnO2 via X-ray photoelectron spectroscopy. The rate of the Mn(II) oxidation reaction was fast relative to typical time scales in drinking water treatment, with an estimated second order rate constant of approximately 1 × 104 M−1 s−1 at pH 9.2 and > 9 × 104 M−1 s−1 at pH 6.2. In general, ferrate is a potential option for Mn(II) oxidation in water treatment.
Figure optionsDownload as PowerPoint slide
Journal: Chemosphere - Volume 159, September 2016, Pages 457–464