| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 4407640 | 1618818 | 2016 | 8 صفحه PDF | دانلود رایگان |
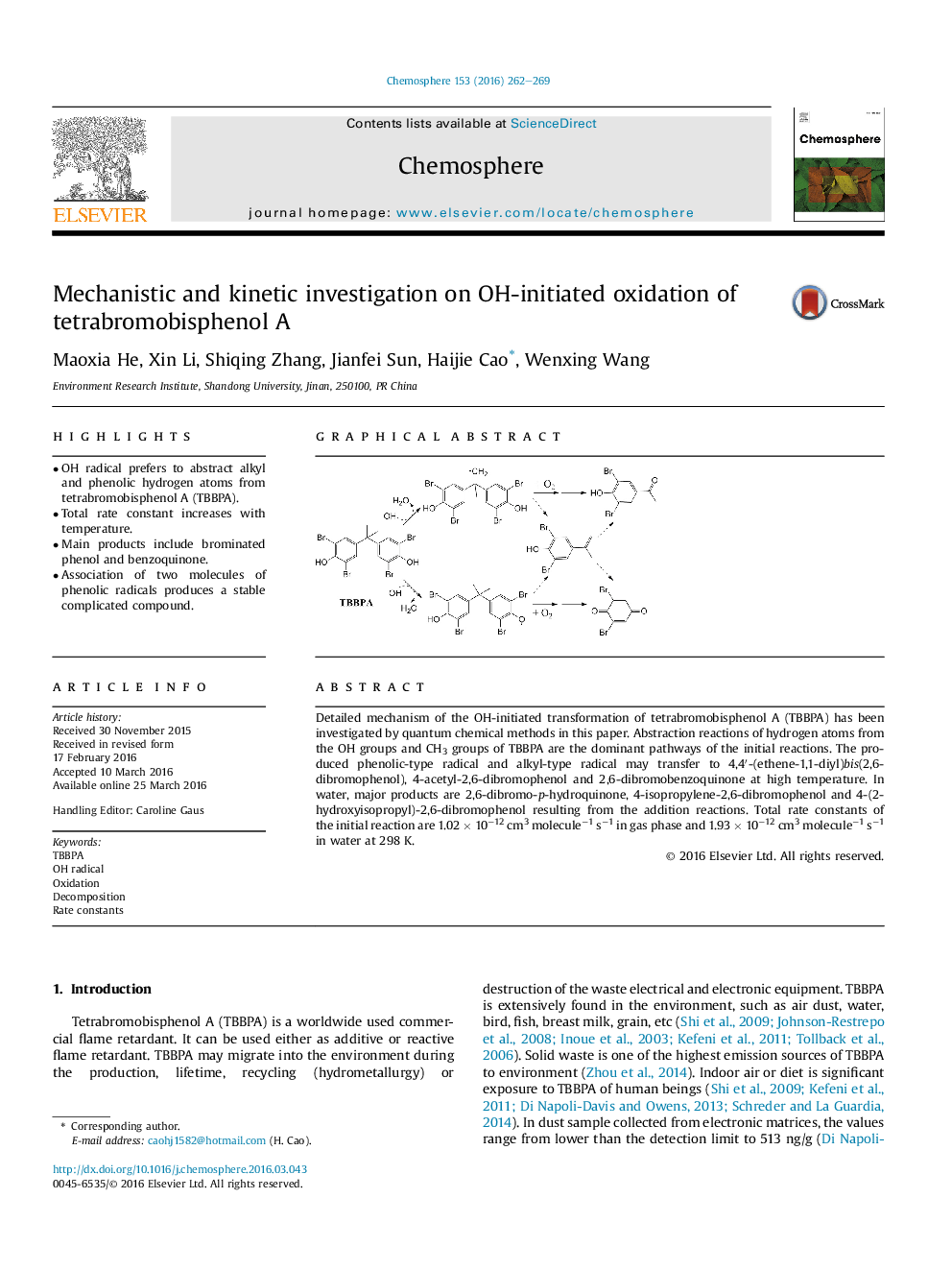
• OH radical prefers to abstract alkyl and phenolic hydrogen atoms from tetrabromobisphenol A (TBBPA).
• Total rate constant increases with temperature.
• Main products include brominated phenol and benzoquinone.
• Association of two molecules of phenolic radicals produces a stable complicated compound.
Detailed mechanism of the OH-initiated transformation of tetrabromobisphenol A (TBBPA) has been investigated by quantum chemical methods in this paper. Abstraction reactions of hydrogen atoms from the OH groups and CH3 groups of TBBPA are the dominant pathways of the initial reactions. The produced phenolic-type radical and alkyl-type radical may transfer to 4,4′-(ethene-1,1-diyl)bis(2,6-dibromophenol), 4-acetyl-2,6-dibromophenol and 2,6-dibromobenzoquinone at high temperature. In water, major products are 2,6-dibromo-p-hydroquinone, 4-isopropylene-2,6-dibromophenol and 4-(2-hydroxyisopropyl)-2,6-dibromophenol resulting from the addition reactions. Total rate constants of the initial reaction are 1.02 × 10−12 cm3 molecule−1 s−1 in gas phase and 1.93 × 10−12 cm3 molecule−1 s−1 in water at 298 K.
Figure optionsDownload as PowerPoint slide
Journal: Chemosphere - Volume 153, June 2016, Pages 262–269