| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 4409094 | 1307463 | 2013 | 8 صفحه PDF | دانلود رایگان |
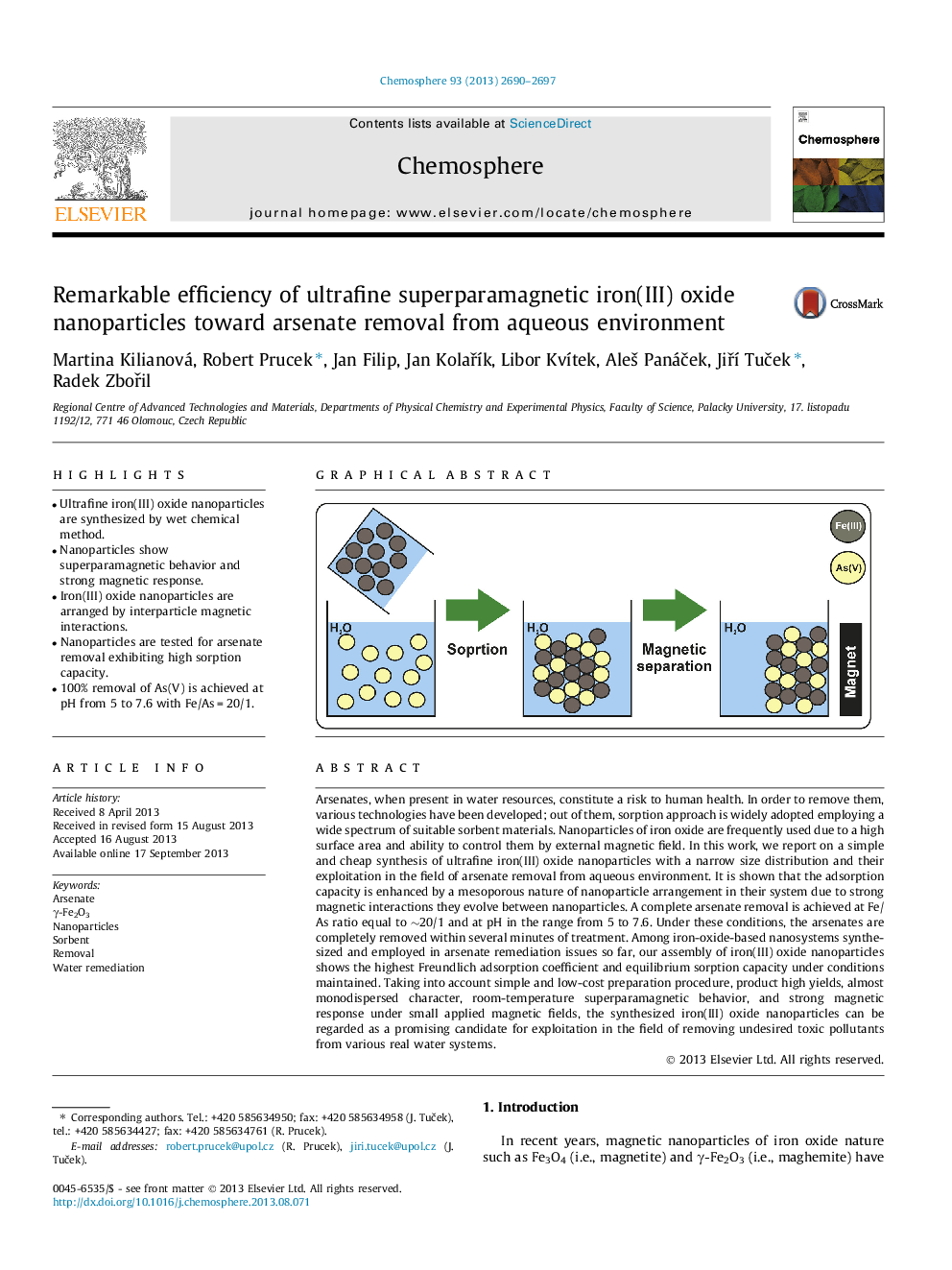
• Ultrafine iron(III) oxide nanoparticles are synthesized by wet chemical method.
• Nanoparticles show superparamagnetic behavior and strong magnetic response.
• Iron(III) oxide nanoparticles are arranged by interparticle magnetic interactions.
• Nanoparticles are tested for arsenate removal exhibiting high sorption capacity.
• 100% removal of As(V) is achieved at pH from 5 to 7.6 with Fe/As = 20/1.
Arsenates, when present in water resources, constitute a risk to human health. In order to remove them, various technologies have been developed; out of them, sorption approach is widely adopted employing a wide spectrum of suitable sorbent materials. Nanoparticles of iron oxide are frequently used due to a high surface area and ability to control them by external magnetic field. In this work, we report on a simple and cheap synthesis of ultrafine iron(III) oxide nanoparticles with a narrow size distribution and their exploitation in the field of arsenate removal from aqueous environment. It is shown that the adsorption capacity is enhanced by a mesoporous nature of nanoparticle arrangement in their system due to strong magnetic interactions they evolve between nanoparticles. A complete arsenate removal is achieved at Fe/As ratio equal to ∼20/1 and at pH in the range from 5 to 7.6. Under these conditions, the arsenates are completely removed within several minutes of treatment. Among iron-oxide-based nanosystems synthesized and employed in arsenate remediation issues so far, our assembly of iron(III) oxide nanoparticles shows the highest Freundlich adsorption coefficient and equilibrium sorption capacity under conditions maintained. Taking into account simple and low-cost preparation procedure, product high yields, almost monodispersed character, room-temperature superparamagnetic behavior, and strong magnetic response under small applied magnetic fields, the synthesized iron(III) oxide nanoparticles can be regarded as a promising candidate for exploitation in the field of removing undesired toxic pollutants from various real water systems.
Figure optionsDownload as PowerPoint slide
Journal: Chemosphere - Volume 93, Issue 11, November 2013, Pages 2690–2697