| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 4409308 | 1307477 | 2013 | 6 صفحه PDF | دانلود رایگان |
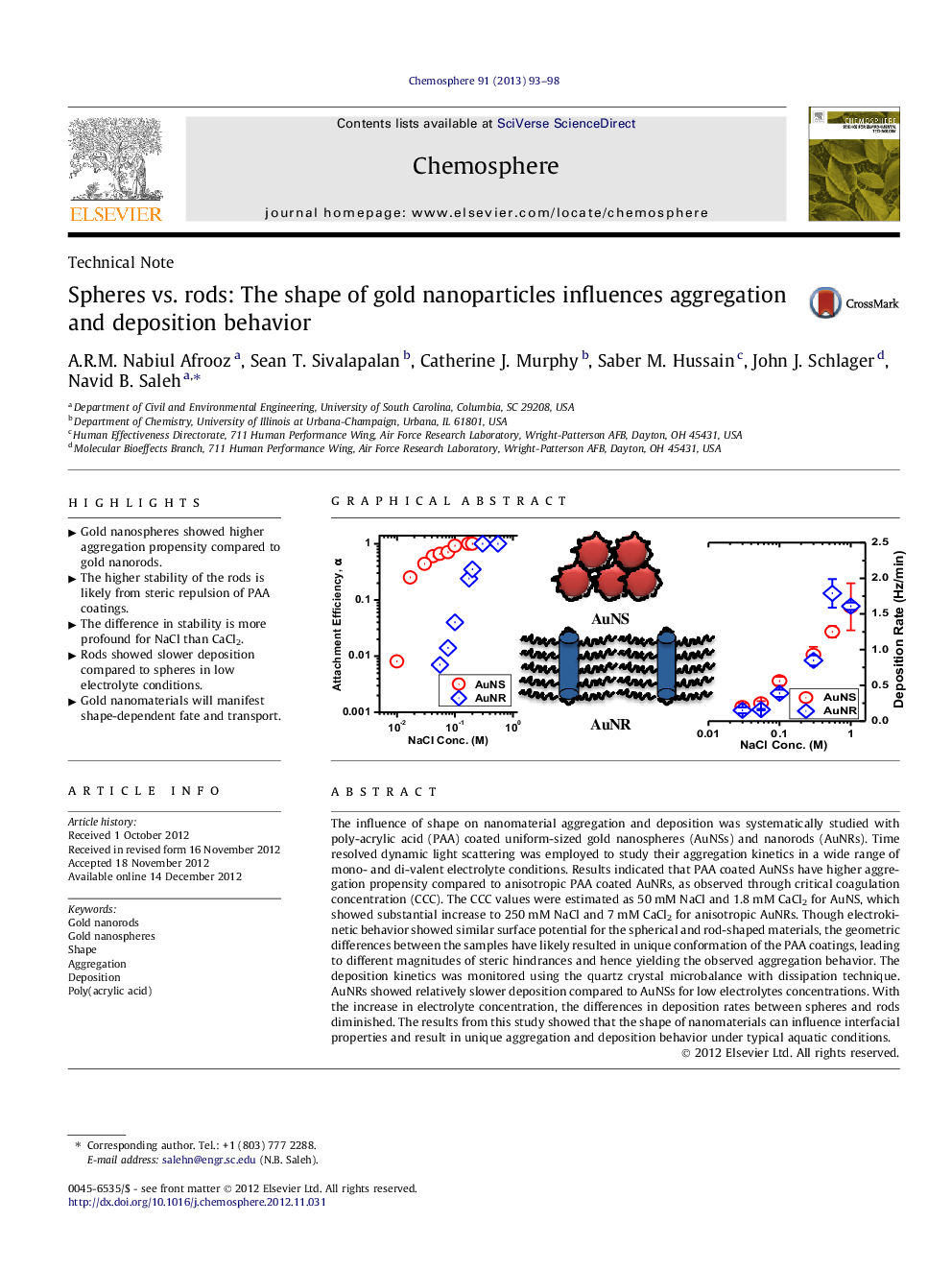
The influence of shape on nanomaterial aggregation and deposition was systematically studied with poly-acrylic acid (PAA) coated uniform-sized gold nanospheres (AuNSs) and nanorods (AuNRs). Time resolved dynamic light scattering was employed to study their aggregation kinetics in a wide range of mono- and di-valent electrolyte conditions. Results indicated that PAA coated AuNSs have higher aggregation propensity compared to anisotropic PAA coated AuNRs, as observed through critical coagulation concentration (CCC). The CCC values were estimated as 50 mM NaCl and 1.8 mM CaCl2 for AuNS, which showed substantial increase to 250 mM NaCl and 7 mM CaCl2 for anisotropic AuNRs. Though electrokinetic behavior showed similar surface potential for the spherical and rod-shaped materials, the geometric differences between the samples have likely resulted in unique conformation of the PAA coatings, leading to different magnitudes of steric hindrances and hence yielding the observed aggregation behavior. The deposition kinetics was monitored using the quartz crystal microbalance with dissipation technique. AuNRs showed relatively slower deposition compared to AuNSs for low electrolytes concentrations. With the increase in electrolyte concentration, the differences in deposition rates between spheres and rods diminished. The results from this study showed that the shape of nanomaterials can influence interfacial properties and result in unique aggregation and deposition behavior under typical aquatic conditions.
Figure optionsDownload as PowerPoint slideHighlights
► Gold nanospheres showed higher aggregation propensity compared to gold nanorods.
► The higher stability of the rods is likely from steric repulsion of PAA coatings.
► The difference in stability is more profound for NaCl than CaCl2.
► Rods showed slower deposition compared to spheres in low electrolyte conditions.
► Gold nanomaterials will manifest shape-dependent fate and transport.
Journal: Chemosphere - Volume 91, Issue 1, March 2013, Pages 93–98