| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 4981701 | 1453840 | 2017 | 8 صفحه PDF | دانلود رایگان |
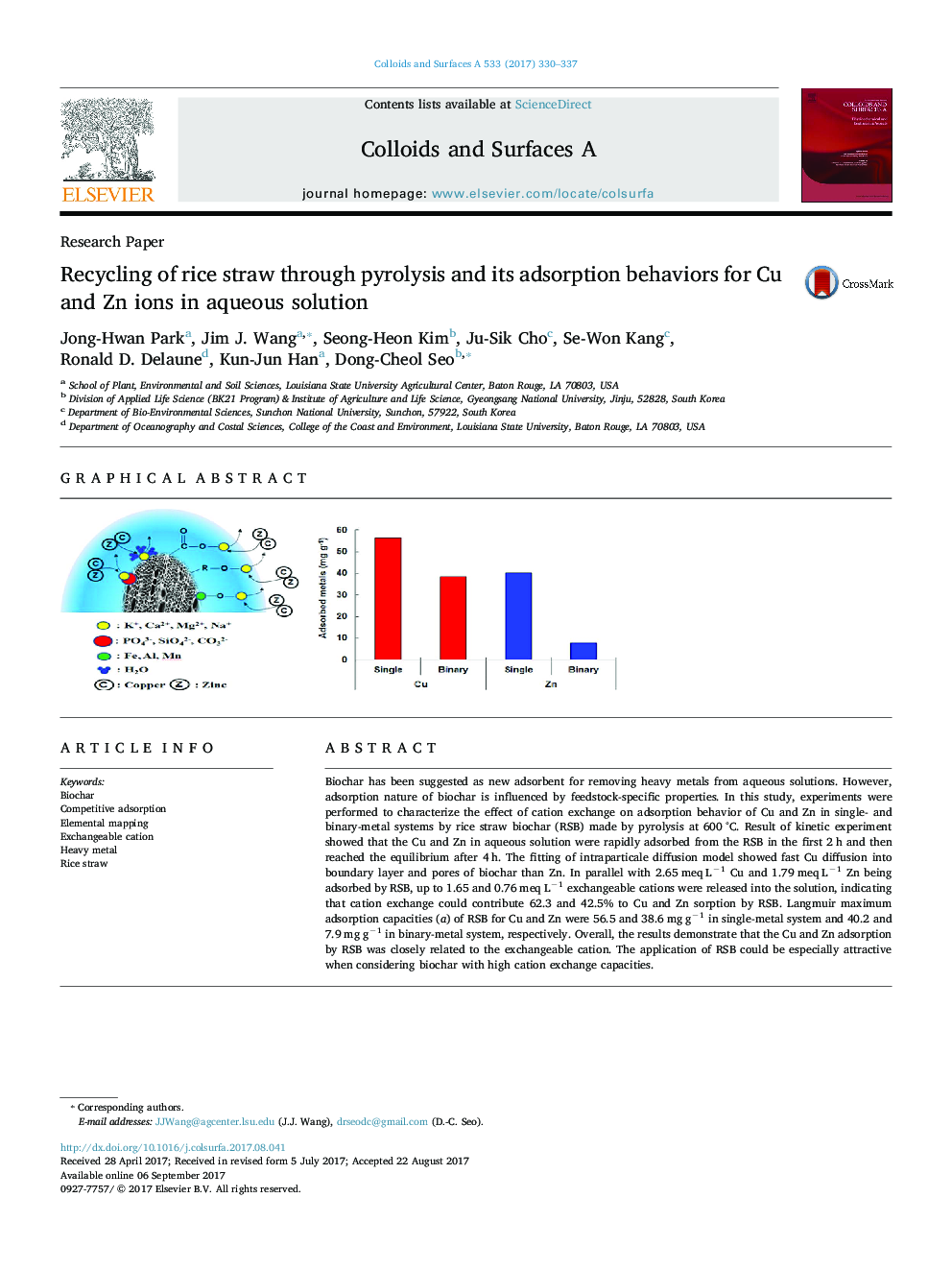
Biochar has been suggested as new adsorbent for removing heavy metals from aqueous solutions. However, adsorption nature of biochar is influenced by feedstock-specific properties. In this study, experiments were performed to characterize the effect of cation exchange on adsorption behavior of Cu and Zn in single- and binary-metal systems by rice straw biochar (RSB) made by pyrolysis at 600 °C. Result of kinetic experiment showed that the Cu and Zn in aqueous solution were rapidly adsorbed from the RSB in the first 2 h and then reached the equilibrium after 4 h. The fitting of intraparticale diffusion model showed fast Cu diffusion into boundary layer and pores of biochar than Zn. In parallel with 2.65 meq Lâ1 Cu and 1.79 meq Lâ1 Zn being adsorbed by RSB, up to 1.65 and 0.76 meq Lâ1 exchangeable cations were released into the solution, indicating that cation exchange could contribute 62.3 and 42.5% to Cu and Zn sorption by RSB. Langmuir maximum adsorption capacities (a) of RSB for Cu and Zn were 56.5 and 38.6 mg gâ1 in single-metal system and 40.2 and 7.9 mg gâ1 in binary-metal system, respectively. Overall, the results demonstrate that the Cu and Zn adsorption by RSB was closely related to the exchangeable cation. The application of RSB could be especially attractive when considering biochar with high cation exchange capacities.
174
Journal: Colloids and Surfaces A: Physicochemical and Engineering Aspects - Volume 533, 20 November 2017, Pages 330-337