| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5130745 | 1490854 | 2017 | 11 صفحه PDF | دانلود رایگان |
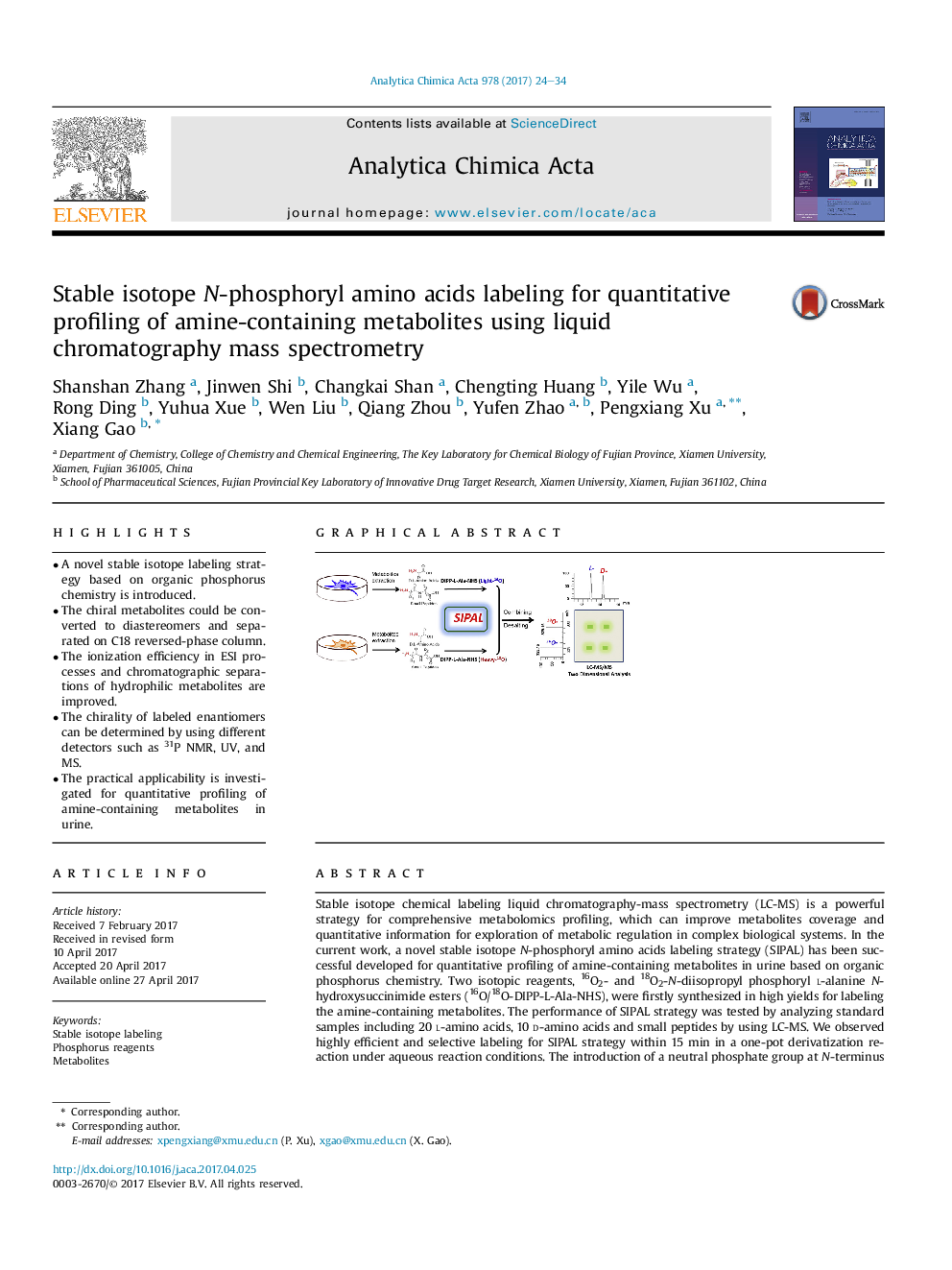
- A novel stable isotope labeling strategy based on organic phosphorus chemistry is introduced.
- The chiral metabolites could be converted to diastereomers and separated on C18 reversed-phase column.
- The ionization efficiency in ESI processes and chromatographic separations of hydrophilic metabolites are improved.
- The chirality of labeled enantiomers can be determined by using different detectors such as 31P NMR, UV, and MS.
- The practical applicability is investigated for quantitative profiling of amine-containing metabolites in urine.
Stable isotope chemical labeling liquid chromatography-mass spectrometry (LC-MS) is a powerful strategy for comprehensive metabolomics profiling, which can improve metabolites coverage and quantitative information for exploration of metabolic regulation in complex biological systems. In the current work, a novel stable isotope N-phosphoryl amino acids labeling strategy (SIPAL) has been successful developed for quantitative profiling of amine-containing metabolites in urine based on organic phosphorus chemistry. Two isotopic reagents, 16O2- and 18O2-N-diisopropyl phosphoryl l-alanine N-hydroxysuccinimide esters (16O/18O-DIPP-L-Ala-NHS), were firstly synthesized in high yields for labeling the amine-containing metabolites. The performance of SIPAL strategy was tested by analyzing standard samples including 20Â l-amino acids, 10 d-amino acids and small peptides by using LC-MS. We observed highly efficient and selective labeling for SIPAL strategy within 15Â min in a one-pot derivatization reaction under aqueous reaction conditions. The introduction of a neutral phosphate group at N-terminus can increase the proton affinity and overall hydrophobicity of targeted metabolites, leading to the better ionization efficiency in electrospray ionization processes and chromatographic separations of hydrophilic metabolites on reversed-phase column. Furthermore, the chiral metabolites, such as d-amino acids, could be converted to diastereomers after SIPAL and successfully separated on regular reversed-phase column. The chirality of labeled enantiomers can be determined by using different detection methods such as 31P NMR, UV, and MS, demonstrating the potential application of SIPAL strategy. In addition, absolute quantification of chiral metabolites in biological samples can be easily achieved by using SIPAL strategy. For this purpose, urine samples collected from a healthy volunteer were analyzed by using LC-ESI-Orbitrap MS. Over 300 pairs of different amine-containing metabolites have been manually identified with high relative abundance (signal-to-noise ratios greater than 10). Finally, a standard peptide could be relatively quantified by using SIPAL strategy in combination with MALDI-TOF MS, suggesting the potential application of this strategy for quantitative proteomics.
212
Journal: Analytica Chimica Acta - Volume 978, 25 July 2017, Pages 24-34